Volume 11, Number 1—January 2005
Dispatch
Plasmodium vivax Malaria
Abstract
We report 11 cases of severe Plasmodium vivax malaria in Bikaner (western India). Patients exhibited cerebral malaria, renal failure, circulatory collapse, severe anemia, hemoglobinurea, abnormal bleeding, acute respiratory distress syndrome, and jaundice. Peripheral blood microscopy, parasite antigen–based assays, and parasite 18s rRNA gene–based polymerase chain reaction showed the presence of P. vivax and absence of P. falciparum.
Plasmodium vivax malaria is prevalent in many regions of the world. It accounts for more than half of all malaria cases in Asia and Latin America. Despite the high prevalence of disease caused by this parasite, research into its effects has lagged disproportionately (1).
Organ dysfunction seen in P. falciparum malaria is not seen in P. vivax infections. Thus, severe malaria is reported with P. falciparum but not with P. vivax infection. If a patient with P. vivax exhibits severe malaria, the infection is presumed to be mixed. When patients have a mixed infection, P. vivax may lessen the effect of P. falciparum and cause the disease to be less severe. Luxemburger et al. observed that severe malaria is 4.2 times less common in patients with mixed P. falciparum and P. vivax infections than in those with P. falciparum alone (2).
During the post-rainy season epidemic of malaria from August to December 2003, many persons along the Indonesia–Pakistan border had severe malaria caused by P. vivax. During the last few outbreaks, we made similar observations, but in 2003 the number of cases was comparatively higher. Clinically severe cases and complications of malaria are commonly due to P. falciparum and not to P. vivax. Beg et al. reported a patient from Pakistan with central nervous system (CNS) involvement with P. vivax, in which the diagnosis was confirmed by polymerase chain reaction (PCR) studies. Beg et al. reviewed the P. vivax cases with CNS involvement reported before 2002; however, most were diagnosed by examination of peripheral blood films (PBF) (3).
We searched available literature and could find only isolated reports of severe P. vivax malaria with cerebral malaria, thrombocytopenia, disseminated intravascular coagulation (DIC), acute respiratory distress syndrome (ARDS), and renal involvement caused by P. vivax. In most cases, the diagnosis was made by PBF examination without molecular diagnostic confirmation, thus allowing for potential errors in species diagnosis (4–13). Although detection of P. vivax in PBF is the standard, its presence does not rule out undetected mixed infection. To rule out this possibility, all the patients received a thorough diagnostic evaluation, which included PBF examination, a rapid diagnostic test for malaria (OptiMAL test, DiaMed AG, Switzerland, which is based on detecting specific Plasmodium LDH antigen by using monoclonal antibody directed against isoforms of the enzyme), and PCR. Our findings are shown in Tables 1 and 2.
All patients were admitted to an intensive care ward dedicated to malaria control. Clinical, biochemical, and radiologic examinations were conducted to establish the diagnosis. Severe malaria was categorized and a treatment regimen of intravenous quinine was instituted according to World Health Organization guidelines (14). Formal approval of the hospital’s ethical committee and consent of the patients were obtained for further studies.
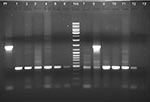
The PCR studies were targeted against the 18S rRNA gene of the parasite and were based on conditions reported earlier (15) utilizing 1 genus-specific 5′ primer and 2 species-specific 3′ primers in the same reaction cocktail. Some of the primer sequences were modified for this study: 1) 5′ATCAGCTTTTGATGTTAGGGT ATT 3′–genus specific, 2) 5′ TAACAAGGACTTCCAAGC–P. vivax specific, and 3) 5′GCTCAAAGATACAAATATAAGC 3′–P. falciparum specific (Figure). Our PCR results in each sample ruled out the possibility of coinfection with P. falciparum. Each sample was subjected to a minimum of 4 rounds of PCR with varying template amounts to eliminate the possibility of overlooking P. falciparum coinfection. In this report, we have not included 2 samples that showed P. vivax infection in PBF examination but showed evidence of mixed infection in PCR examination. The result of PCR analysis of 1 sample is shown in lane 8 of the Figure.
The essential pathologic feature of severe malaria is sequestration of erythrocytes that contain mature forms of the parasite in the deep vascular beds of vital organs, thus producing cerebral malaria, renal failure, hepatic dysfunction, or ARDS. However, severe anemia and thrombocytopenia that causes bleeding diathesis is produced by hemolysis, reduced cell deformity of parasitized and nonparasitized erythrocytes, increased splenic clearance, reduction of platelet survival, decreased platelet production, and increased splenic uptake of platelets, and can be produced by P. vivax and P. falciparum infection. Our clinical data from these patients strongly indicate that P. vivax can cause both sequestration-related and nonsequestration-related complications of severe malaria, including cerebral malaria, renal failure, circulatory collapse, severe anemia, hemoglobinurea, abnormal bleeding, ARDS, and jaundice, all of which are commonly associated with P. falciparum infections. None of the patients described in this study had evidence of P. falciparum infection at the level of antigen (parasite LDH) and 18S rRNA–based PCR test, apart from PBF examination.
This is the first detailed report of severe P. vivax malaria. We cannot comment on a pathogenic mechanism causing multiple organ dysfunction and the characteristics of host-parasite interrelationship responsible for it. A detailed prospective study is required to address these issues.
Dr. Kochar is professor and head of the Department of Medicine at S.P. Medical College, Bikaner, India. He is currently engaged in clinical research on malaria.
Acknowledgments
We thank Altaf A Lal and Subrata Sinha for stimulating insights and discussions; Birla Institute of Technology and Science, Pilani, for providing facilities for the investigation; and S.P. Medical College and Associated Group of Hospitals, Bikaner, for diagnosing the cases and caring for the patients.
Vishal Saxena received fellowship from the Council of Scientific and Industrial Research.
References
- Luxemburger C, Ricci F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–62. DOIPubMedGoogle Scholar
- Beg MA, Khan R, Baig SM, Gulzar Z, Hussain R, Smego RA. Cerebral involvement in benign tertian malaria. Am J Trop Med Hyg. 2002;67:230–2.PubMedGoogle Scholar
- Verma KC, Magotra ML. Vivax cerebral malaria in Jammu. Indian Pediatr. 1976;13:229–31.PubMedGoogle Scholar
- Valecha N, Bagga A, Chandra J, Sharma D. Cerebral symptoms with P. vivax malaria. Indian Pediatr. 1992;29:1176–7.PubMedGoogle Scholar
- Patial RK, Kapoor D, Mokta JK. Cerebral dysfunction in vivax malaria: a case report. Indian J Med Sci. 1998;52:159–60.PubMedGoogle Scholar
- Kakar A, Bhoi S, Prakash V, Kakar S. Profound thrombocytopenia in Plasmodium vivax malaria. Diagn Microbiol Infect Dis. 1999;35:243–4. DOIPubMedGoogle Scholar
- Mehta KS, Halankar AR, Makwana PD, Torane PP, Satija PS, Shah VB. Severe acute renal failure in malaria. Postgrad Med. 2001;47:24–6.PubMedGoogle Scholar
- Tanious MA, Kogelman L, McGovern B, Hassoun PM. Acute respiratory distress syndrome complicating Plasmodium vivax malaria. Crit Care Med. 2001;29:665–7. DOIPubMedGoogle Scholar
- Makkar RP, Monga SM, Gupta AK. Plasmodium vivax malaria presenting with severe thrombocytopenia. Braz J Infect Dis. 2002;6:263–5. DOIPubMedGoogle Scholar
- Mohapatra MK, Padhiary KN, Mishra DP, Sethy G. Atypical manifestations of Plasmodium vivax malaria. Indian J Malariol. 2002;39:18–25.PubMedGoogle Scholar
- Jadhav UM, Patkar VS, Kadam NN. Thrombocytopenia in malaria—correlation with type and severity of malaria. JAPI. 2004;52:615–8.PubMedGoogle Scholar
- World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94:38–40.
- Das A, Holloway B, Collins WE, Sharma VP, Ghosh SK, Sinha S, Species-specific 18S rRNA gene amplification for the detection of P. falciparum and P. vivax malaria parasites. Mol Cell Probes. 1995;9:161–5. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 11, Number 1—January 2005
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Dhanpat K. Kochar, C-54, Sadul Ganj, Bikaner – 334003 India; fax: 0091-151-2201502
Top