Volume 11, Number 6—June 2005
Dispatch
Community-acquired Methicillin-resistant Staphylococcus aureus, Uruguay
Abstract
A novel, methicillin-resistant Staphylococcus aureus clone (Uruguay clone) with a non–multidrug-resistant phenotype caused a large outbreak, including 7 deaths, in Montevideo, Uruguay. The clone was distinct from the highly virulent community clone represented by strain MW2, although both clones carried Panton-Valentine leukocidin gene and cna gene.
Since the 1990s, methicillin-resistant Staphylococcus aureus (MRSA) infections have been increasingly recognized in the community, and MRSA strains isolated from patients with community-associated cases have been called community-associated MRSA (CA-MRSA) (1). CA-MRSA strains have been reported to differ from isolates from hospitals (healthcare-associated MRSA; HA-MRSA) in many characteristics such as susceptibility to antimicrobial drugs, types of staphylococcal cassette chromosome (SCC) mec element, and repertoires of exotoxin gene. In Uruguay, MRSA strains are among the most prevalent nosocomial pathogens. In late 2001, we observed a case in a young man with recurrent boils who visited an outpatient clinic. An MRSA strain that was susceptible to other drugs was isolated from the patient. After that, pediatric infections associated with similar strains were observed (2). The initial sporadic cases were followed by an epidemic increase of infections in the community, hospitals, and jails. We began to record the microbiologic data and analyze cases together with the National Antimicrobial Resistance Surveillance Network belonging to the Public Health Ministry in Uruguay, and we concluded that a large outbreak of CA-MRSA strains occurred in Uruguay. Here we report the emergence of a novel CA-MRSA clone, which has been shown by multilocus sequence typing (MLST) and SCCmec type to be distinct from the midwestern CA-MRSA strain.
We studied patients with non–multidrug-resistant MRSA infections identified at 2 hospital centers in the metropolitan area of Montevideo, Uruguay, Hospital Maciel and Centro de Asistencia del Sindicato Médico del Uruguay, from January 2002 to October 30, 2003. A total of 125 S. aureus strains that were resistant to oxacillin alone or to erythromycin in addition were isolated from outpatients and inpatients. Since 1 of our members noticed some cases of pyogenic infections in a prison, we conducted a sentinel study of skin and soft tissue infection (SSTI) in the 2 main prisons from May to June in 2003. We isolated 40 non–multidrug-resistant MRSA strains from 58 inmates with SSTIs. Of these 40 strains, 17 were randomly selected to be analyzed. Susceptibilities to 8 antimicrobial drugs (oxacillin, vancomycin, gentamicin, rifampin, ciprofloxacin, erythromycin, clindamycin, and trimethoprim-sulfamethoxazole) were tested by the Kirby Bauer disk diffusion test (Becton Dickinson, Cockeysville, MD, USA). Production of PBP2´ and protein A were verified by MRSA Screen latex PBP2´ (Denka Seiken-Oxoid Ltd, London, UK) and latex slide agglutination kits (Oxoid, Hampshire, UK), respectively. Most (133/142, 94%) showed heterogeneity in the degree of resistance to oxacillin, since double halos or haze zones were observed around the disk containing 1 μg of oxacillin.
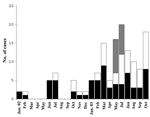
The course of the outbreak during the 22 months is shown in Figure 1. The number of MRSA infections increased greatly in 2003. The Table summarizes the cases in which non–multidrug-resistant MRSA strains were isolated. Of the 125 case-patients, 112 were adults. The mean age was 39.7 years, which was lower than that of case-patients infected with HA-MRSA strains (mean age 59 years) reported previously (3). We classified the cases as community-associated if MRSA was isolated from cultures performed within 48 hours after admission to hospitals and excluded patients who had previously noted criteria for risk factors of HA-MRSA acquisition: recent hospitalization (within the last 6 months); use of medical devices (such as a permanent indwelling catheter or percutaneous medical device); exposure to healthcare services, including invasive or surgical procedures; residence in a long-term care facility; and any known antimicrobial drug use within the past year (4,5). Community-associated cases were dominant (78%). The predominant infection type in adults was skin and soft tissue infection (n = 86) such as abscesses, boils, and cellulitis, followed by respiratory tract infections, among which 12 of 14 were pneumonia. Four of 14 adult patients with respiratory tract infections exhibited symptoms of acute severe pneumonia, with histopathologic findings of "necrotizing pneumonia," and all died after bacteremia developed. Besides developing in these 4 patients with fatal cases, bacteremia developed in 9 other patients, and 3 of them died. The sites of infection preceding the bacteremia for these 3 patients were skin and soft tissue, bone and joint (septic arthritis), and unknown (classified as septic syndrome in Table), respectively. Bacteremia also developed in 3 pediatric patients. In total, bacteremia developed in 17 patients, and 7 died during the study period. We studied the molecular microbiologic characteristics of 68 isolates: 16 from the patients in whom bacteremia developed, 35 randomly selected from all case-patients, and 17 from the inmates.
Pulsotypes, coagulase isotypes, SCCmec types, and exotoxin gene repertoires were examined by the methods indicated in the footnotes of the Table A1. Among 6 pulsotypes identified in the Uruguay strains, 38 (74.5%) of 51 isolates from patients and all 17 isolates from the inmates, had related pulsotypes within 4 band differences designated A1–A4 (Table A1). All of them produced type-4 coagulase and carried type IVc SCCmec element, 53 of 55 carried lukS,F-PV genes, and 51 of 55 carried the cna gene. Isolates from 11 of 16 bacteremic case-patients, including 6 who died, belonged to pulsotype A. Evidence shows that a clone (pulsotype A-SCCmec IVc), which possessed both cna and lukS,F-PV genes, caused the outbreak in Uruguay.
Other pulsotype strains carried primarily other SCCmec elements, such as type-II, type-IVa, or type-V, and produced type-2, -5, or -7 coagulase and did not carry lukS,F-PV genes. Notably, 49 (96%) of 51 strains isolated from both community-associated and healthcare-associated cases carried either type-IV or type-V SCCmec element, which have been found in CA-MRSA strains (6–8).
We compared characteristics of outbreak strains with those of previously investigated CA-MRSA strains isolated in the United States (MW2) and Australia (A803355, A823549, and E802537), and a strain isolated from an outpatient in Japan in 1981 (81/108) (Figure 2). All of them possessed both cna and lukS,F-PV genes as well. The dominant outbreak strains belonged to ST-30, which was the same as Australian and Japanese strains and distinct from MW2 (ST-1). In addition, we found that the pulsotype of strain A803355 (reported previously as H1) was identical to pulsotype A1, the most representative pulsotype among tested strains. Pulsotypes of other 2 Australian strains, A823549 and E8025347, were classified into the same cluster as A, while that of 81/108 showed a similar type on pulsed-field gel electrophoresis (PFGE) to pulsotype A. In contrast, only 2 Uruguay isolates,UR20 and UR41, had a PFGE pattern similar to that of MW2.
The outbreak of CA-MRSA in Uruguay involved >1,000 patients and ≤12 deaths, when the data after this study period are added. According to a follow-up survey conducted at jails from May to October 2003, 890 of 1,142 inmates were infected with similar pyogenic infections after an outbreak of scabies (10). Five patients required hospitalization. Boils and abscesses in the buttocks and neck were the most prevalent infections (85%), followed by hidradenitis and cellulitis. The prevalence of a new clone represented by UR 6 (Uruguay clones) is considered to have been the cause of this large outbreak. We are conducting a further study of isolates from patients after this study period and isolates from inmates in a follow-up survey, and a final conclusion awaits those results.
We have suggested 2 genes as important candidates for high virulence in the midwestern CA-MRSA strains represented by strain MW2 (11), which is distributed in the United States and Europe (12,13). Since the Uruguay clone shared lukS,F-PV genes and cna gene with the midwestern CA-MRSA clone, this study strengthened the likelihood that these 2 genes are contributors for high virulence. Their genotypes, however, were completely different, and we now appear to have 2 distinct clones of highly virulent CA-MRSA. That certain CA-MRSA strains, identified in Australia as carrying the luk-PV and cna genes, had an identical PFGE pattern with UR6 does not imply that the same CA-MRSA clone has been disseminated between Uruguay and Australia because these 2 clones had distinct SCCmec elements, IVc and IVa, respectively. Since the MRSA clone originated when the SCCmec was integrated into the chromosome of a S. aureus strain, the 2 MRSA clones are understood to have originated independently by acquiring different SCCmec genes in their respective countries (14,15). In this regard, the difference in the SCCmec type was not reflected in the PFGE pattern. This example provides an excellent illustration of the fact that clonality cannot be judged on PFGE pattern alone.
Nonetheless, CA-MRSA strains with identical PFGE and MLST patterns possessing lukS,F-PV genes and cna genes exist in both Uruguay and Australia. This finding may indicate the existence of a genetically stable, virulent, multidrug-susceptible S. aureus (MSSA) clone in a community that extends beyond country borders and across the ocean. The MSSA clone, which has lukS,F-PV and cna genes, is established in the community and occasionally acquires SCCmec when the use of β-lactam antimicrobial drugs is increased to a stressful level for the survival of the MSSA clone in the community. Since we have also found MRSA strains isolated in the 1980s in Japan with a similar genotype to that of UR6 and possessing PVL and cna genes, the outbreak observed in Uruguay could occur in any part of the world if social or medical predisposing conditions are met.
Dr. Ma is an associate research fellow in the Department of Bacteriology, Juntendo University, Tokyo, Japan. Her research focuses on community-acquired methicillin-resistant S. aureus.
Acknowledgments
We thank G. Bogliaccini, A. Galiana Villar, M. Dibarboure, T. Pais, M. Moulia, and G. Navas. We also thank Tsai-Ling Yang Lauderdale for her kind help in preparing manuscript.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (13226114) and a Grant-in-Aid for the 21st Century Center of Excellence program from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- Eady EA, Cove JH. Staphylococcal resistance revisited: community-acquired methicillin resistant Staphylococcus aureus—an emerging problem for the management of skin and soft tissue infections. Curr Opin Infect Dis. 2003;16:103–24. DOIPubMedGoogle Scholar
- Galiana VA. Infecciones por Staphylococcus aureus adquiridos en la comunidad. Arch Pediatr Urug. 2003;74:26–9.
- Annual report: National Committee of Hospital Infection Control. Montevideo, Uruguay: Ministry of Public Health; 2001.
- Gorak EJ, Yamada SM, Brown JD. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin Infect Dis. 1999;29:797–800. DOIPubMedGoogle Scholar
- Shopsin B, Mathema B, Martinez J, Ha E, Campo ML, Fierman A, Prevalence of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the community. J Infect Dis. 2000;182:359–62. DOIPubMedGoogle Scholar
- Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–52. DOIPubMedGoogle Scholar
- Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O'Brien FG, Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. DOIPubMedGoogle Scholar
- Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637–51. DOIPubMedGoogle Scholar
- Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin- resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15.PubMedGoogle Scholar
- Mowszowicz M, Pedreira W, Galiana A, Hiramatsu K, Ma XX, Ito T, Efficacy of co-trimoxazole–high dosage short course (SXT-HDSC) in community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) outbreak in Uruguay jails 2003. Int J Infect Dis. 2004;8(Suppl 1):185.
- Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–27. DOIPubMedGoogle Scholar
- Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. DOIPubMedGoogle Scholar
- Diep BA, Sensabaugh GF, Somboona NS, Carleton HA, Perdreau-Remington F. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J Clin Microbiol. 2004;42:2080–4. DOIPubMedGoogle Scholar
- Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001;9:486–93. DOIPubMedGoogle Scholar
- Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist Updat. 2003;6:41–52. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 11, Number 6—June 2005
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Keiichi Hiramatsu, Department of Bacteriology, Juntendo University, 2-1-1 Hongo, Bunkyo-ku, Tokyo, Japan 113-8421; fax: 81-3-5684-7830
Top