Volume 12, Number 9—September 2006
Research
Extrapulmonary Tuberculosis by Nationality, the Netherlands, 1993–2001
Abstract
This study describes the epidemiology of extrapulmonary tuberculosis (TB) in the Netherlands from 1993 through 2001. We assessed whether the increasing numbers of inhabitants with a non-Western ethnic background had an effect on the number of extrapulmonary patients. We used data from the Netherlands Tuberculosis Register and included all cases of TB diagnosed in the Netherlands between January 1, 1993, and December 31, 2001. Information on age, sex, nationality, year of diagnosis, culture result, anatomic location of the site of disease, and HIV status was retrieved from the register. Of 13,258 patients with TB, 8,216 (62%) had pulmonary TB, and 5,042 (38%) had extrapulmonary TB. Non-Dutch nationals were more likely to have most types of extrapulmonary TB. The growth of the number of inhabitants with a non-Western ethnic background in the Netherlands explains the proportional growth of extrapulmonary TB. Physicians need to be aware of the changing clinical picture of TB.
Tuberculosis (TB) is a major public health problem, affecting 8 million persons per year worldwide (1). The global incidence rate of TB per capita is growing by ≈1.1% per year (1). Contrary to the increasing number of TB cases in developing countries, the number of cases in industrialized countries is stable or decreasing (2–4). In the United States, a decreasing trend of the total number of TB patients is seen with an increasing proportion of TB cases with extrapulmonary TB, resulting in a rising proportion from 7.8% in 1964 to 20% in 2001 (5–8). Both the HIV epidemic and changes in population demographics, with rising numbers of immigrants, are being held responsible for this proportional increase of extrapulmonary TB (6,7,9). A recent study of extrapulmonary TB in Hong Kong Special Administrative Region, People's Republic of China showed that 22.3% of the TB cases were extrapulmonary (10), while a small Canadian study found a proportion of 46% (11). No national studies about extrapulmonary TB in developing countries are known.
Extrapulmonary TB refers to TB outside the lungs. Mycobacteria may spread through lymphatic or hematogenous dissemination to any tract or through coughing and swallowing to the gastrointestinal tract. Bacteria may remain dormant for years at a particular site before causing disease. Since extrapulmonary TB can affect virtually all organs, it has a wide variety of clinical manifestations, which causes difficulty and delay in diagnosis (7,8). Obtaining material for culture confirmation of extrapulmonary TB is much more difficult than obtaining material for culture confirmation of pulmonary TB (7). Extrapulmonary TB is more often diagnosed in women and young patients (6,7,9–13). In the United States, extrapulmonary TB is associated with ethnic minorities and those born in other countries (6). In many countries, patients from Asian origin are known to have a higher incidence of extrapulmonary TB, especially lymphatic TB (11,14–17). A study of Somali TB patients in Minnesota showed frequent lymphatic TB as well (18). In HIV-infected patients the frequency of extrapulmonary TB depends on the degree of decreased cellular immunity (19,20). In patients with <100 CD4 cells/mL, extrapulmonary and disseminated TB counts for 70% of all forms of TB (21).
The Netherlands has a low incidence of HIV and, similar to other industrialized countries, a decreasing TB incidence rate. TB was a common disease in the nineteenth century, but after the Second World War the incidence rate declined rapidly (22). During the last decade, the numbers of inhabitants with a non-Western ethnic background has been growing (23). The proportion of TB patients with a non-Dutch nationality has increased from 30% in 1980 to 63% in 2000 (22,24,25). Dutch policy regulates the screening by chest radiograph of immigrants from countries with a high prevalence who intend to stay for more than 3 months (26). No studies have described the epidemiology of extrapulmonary TB in the Netherlands, with the exception of 1 study on bone and joint TB (27).
This article describes the epidemiology of extrapulmonary TB in the Netherlands between 1993 and 2001. Our main focus is on the relation between extrapulmonary TB and nationality. We assess whether the increasing number of inhabitants with a non-Western ethnic background has had an effect on the number of extrapulmonary patients.
For this study, we used data from the Netherlands Tuberculosis Register (NTR), an anonymous case register held by KNCV Tuberculosis Foundation. Every TB patient reported to 1 of the 45 municipal health services in the Netherlands is included in this register. Reporting to the register is voluntary. Comparison with the mandatory notification system of the Ministry of Health suggests 99% completeness (28). We included all cases of TB diagnosed in the Netherlands from January 1, 1993 through December 31, 2001. Information on age, sex, and nationality, as recorded in the passport, year of diagnosis, culture result, anatomic location of the site of disease, and HIV status was retrieved from the register. Non-Dutch nationality was divided into 7 different nationality groups: Europe (central and eastern: Poland, Czech, Slovakia, Hungary, Romania, Bulgaria, Albania and the countries of the former Yugoslavia and the former Soviet Union), Turkey, Morocco, Somalia, Africa (other), Asia, and other countries. HIV testing is not a standard procedure for TB patients in the Netherlands. We considered case-patients with a record of impaired immunity due to HIV infection as HIV infected and all others as HIV status unknown.
Information about the anatomic location of disease was registered using 2 different classification systems. In the NTR, the location of TB is generally divided into 3 categories, pulmonary, extrapulmonary, or combined pulmonary and extrapulmonary disease, and the disease is classified according to the site of the disease by using the International Classification of Diseases 9th revision (ICD-9). We first used the information from the ICD-9 classification to divide the patients into a pulmonary TB group, i.e., major site of disease location inside the lungs, and an extrapulmonary TB group, i.e., the major site of disease was outside the lungs. Thereafter, 8 types of extrapulmonary disease were defined: lymphatic, pleural, bone and/or joint, genitourinary, miliary, peritoneal, meninges and/or central nervous system (CNS), and all other sites combined. If information from the ICD-9 classification was not available, we used the general information about location of the disease. Case-patients with general location pulmonary disease were allocated to the pulmonary TB group and those with extrapulmonary disease were allocated to the extrapulmonary TB group, site of disease unspecified. Patients for whom no information about the ICD-9 classification was available, and with the general location of combined pulmonary and extrapulmonary disease, were excluded from the analysis.
Each patient was included once in our dataset. If a patient was reported to the register with TB more than once from January 1, 1993, through December 31, 2001, only the information from the first registration was included in the dataset. Patients with a culture diagnosis of Mycobacterium bovis and M. bovis BCG were excluded as were patients with missing data for age, sex, or nationality.
We obtained the total number of inhabitants with Dutch and non-Dutch nationality in the Netherlands from January 1, 1993, through January 1, 2002, from the Central Bureau of Statistics of the Netherlands (29). We used these figures as denominators to assess trends in time of the incidence of extrapulmonary and pulmonary TB by Dutch or non-Dutch nationality with Poisson regression. One-way analysis of variance (ANOVA) was used to analyze differences in means of age. We used the χ2 test to compare the proportions of pulmonary and extrapulmonary TB cases and non parametric tests to compare time to TB diagnosis from immigration for foreign born persons. Differences between pulmonary TB and the 8 separate types of extrapulmonary TB, were tested by using logistic regression, with pulmonary disease as the reference category. The variables age, sex, nationality and HIV status were included in a multivariate logistic regression model to adjust for confounding. Statistical analysis was performed by using SPSS version 11.5 for Windows (SPSS Inc, Chicago, IL, USA).
Between January 1993 and December 2001, 13,943 cases of TB were reported to the Netherlands Tuberculosis Register, 10,688 (76.7%) were culture positive. The 270 case-patients that were reregistrations were excluded. Also excluded were 135 patients who had a diagnosis of M. bovis or M. bovis BCG infection; 190 patients with missing information about age, sex, or nationality; and 90 patients with a missing ICD-9 classification and combined pulmonary and extrapulmonary disease.
Of the total of 13,258 (95.1%) TB patients available for analysis, 8,216 (62.0%) had pulmonary TB, and 5,042 (38.0%) had an extrapulmonary major site of disease (Table 1). The proportion with culture confirmation was slightly larger among the pulmonary (71.5%) than among the extrapulmonary TB patients (67.4%) (χ2 test, p<0.01). The male-to-female ratio was 1.5:1. Male TB patients were slightly younger than female TB patients (ANOVA, p<0.01), the mean age for men was 39.0 years and for women, 40.2 years. Most TB patients (57.1%) were non-Dutch. Of the 7,576 non-Dutch patients, 1,692 (22.3%) were Somali, 1,600 (21.1%) were Asian, 1,267 (16.7%) were Moroccan, and 1,245 (16.4%) were African (other). Asians included 32 different nationalities; the largest number of patients was from Pakistan (243 cases), followed by Indonesia (239 patients) and the People's Republic of China (179 patients). The African (other) group consisted of 48 nationalities with the largest numbers of patients being from Ethiopia (163 patients) and Angola (146 patients). Patients from Somalia, Asia, and Morocco more frequently had a diagnosis of extrapulmonary TB then did Dutch patients, whereas patients from central and eastern Europe had extrapulmonary TB less often (χ2 test, p<0.01). Non-Dutch patients were significantly younger than Dutch patients (ANOVA p<0.01), the mean ages were 31.7 and 49.8 years, respectively. The male-to-female ratio was 1.4:1 among Dutch patients and 1.5:1 among non-Dutch patients (χ2 test, p<0.01). Among the non-Dutch, 41.7% of the TB patients had extrapulmonary TB, compared with 33.1% of the Dutch TB patients (p<0.01). No interaction was found between the variables age, nationality, sex, and HIV status.
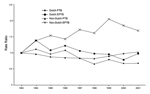
Between 1993 and 2001 the total number of pulmonary TB cases decreased (rate ratio per year 0.96, 95% confidence interval [CI] 0.95–0.96; p<0.01), especially among the Dutch population (Figure). The total number of extrapulmonary patients showed no significant change in time (rate ratio per year 1.00, 95% CI 0.99–1.02; p = 0.40), but the number of extrapulmonary TB patients with a Dutch nationality decreased (rate ratio per year 0.96, 95% CI 0.94–0.98; p<0.01), while the number of extrapulmonary TB patients with a non-Dutch nationality increased (rate ratio per year 1.06, 95% CI 1.04–1.07; p<0.01).
Extrapulmonary TB was relatively more prevalent among female (45.3%) than among male TB patients (33.1%) (p<0.01). The mean age of extrapulmonary TB patients was slightly higher (40.3 years) than that of pulmonary cases (39.0 years; ANOVA p<0.01). The most frequent types of extrapulmonary TB were lymphatic TB (1,963 cases), pleural TB (1,036 cases), and TB of the bones and/or joints (465 cases) (Table 2). Other sites accounted for 111 (TB of meninges and/or CNS) to 246 (miliary TB) cases, while for 379 of the extrapulmonary cases, site of disease was not recorded. The proportion of HIV-infected patients ranged from 0.9% (genitourinary TB) to 16.7% (miliary TB), depending on the site of disease (Table 3). The median time since immigration into the Netherlands to diagnosis of TB among non-Dutch was 36.0 months (mean 73.0 months) and varied from 7.9 months in central and eastern Europeans to 9.0 years in Moroccans. Among all non-Dutch pulmonary TB had a shorter time from immigration to diagnosis (median 24.0 months) than extrapulmonary TB (median 44.6 months) (Mann-Whitney U test p<0.01).
The genitourinary tract was the site of TB that had the smallest proportion of non-Dutch patients (34.2%) while 80.9% of the patients with peritoneal TB were non-Dutch. Somali TB patients had a strongly increased frequency of peritoneal TB (crude odds ratio 9.4, adjusted odds ratio [AOR] 12, 95% CI 7.6–20), lymphatic TB (Crude odds ratio 7.2, AOR 7.8, 95% CI 6.6–9.3) and TB of bones and/or joints (crude odds ratio 3.5, AOR 6.1, 95% CI 4.5–8.3) than the Dutch (Table 4). Asian TB patients had a higher frequency of lymphatic TB (crude odds ratio 4.1, AOR 4.2, 95% CI 3.6–5.0) and peritoneal TB (crude odds ratio 3.2, AOR 3.4, 95% CI 2.0–5.9) than the Dutch. The proportion of TB of the bones and/or joints, genitourinary TB, and miliary TB compared to pulmonary TB increased with the age of the patients. Both the age groups <14 years and 45–64 years were associated with TB of the meninges and/or CNS, in univariate analysis (crude odds ratio 3.9 and 1.8, respectively). When the study population was stratified in <14 and >15 years age groups, all types of extrapulmonary TB were associated with the oldest age group, except for TB of the meninges and/or CNS, which was weakly associated with the youngest age group (AOR 1.7, 95% CI 0.9–3.1). HIV infection was positively associated with miliary TB (AOR 7.4, 95% CI 4.9–11) and TB of the meninges and/or CNS (AOR 3.3, 95% CI 1.6–6.7) and negatively associated with pleural and genitourinary TB.
In the Netherlands, the number of inhabitants with a non-Western ethnic background increased over the past 2 decades (23). The Netherlands is likely to remain an immigration destination for persons from non-Western countries, although changes in immigration laws can change this situation (23,24). We assessed the effect of these immigration patterns on the incidence of pulmonary and extrapulmonary TB.
Between 1993 and 2001, the number of pulmonary TB cases per year declined, whereas the number of extrapulmonary TB cases remained stable, thus showing a proportional increase. This trend is similar to the trend observed in studies in the United States (6,7). Our study shows that a non-Dutch nationality, especially Somali and Asian, was positively associated with extrapulmonary TB when compared with the results for pulmonary TB. This finding suggests that the most likely explanation for the proportional increase of extrapulmonary TB is the growth of the number of inhabitants with a non-Western ethnic background.
Our analysis showed that persons from non-Western national groups, especially Somalis, Asians, and Moroccans, were more likely to receive a diagnosis of most types of extrapulmonary TB than Dutch nationals. When looking at the absolute number of patients with lymphatic and peritoneal TB exclusively, patients with Somali nationality even outnumbered Dutch patients (497 and 58 vs 441 and 34, respectively). In agreement with the literature, we found a strongly positive association between Somali nationality and lymphatic and bone and/or joint TB (18,27) and between Asian nationalities and lymphatic TB (6,10,11,16). Our study demonstrated a statistically significant, strong, positive association between peritoneal TB and Somali, Moroccan, or Asian nationality. In contrast, pleural TB was significant negatively associated with the non-Dutch patients when results were compared with those of the Dutch patients, a finding that we cannot explain.
Several explanations are possible for the association of extrapulmonary TB and a non-Dutch nationality. Non-Dutch persons may have a higher frequency of extrapulmonary TB due to an impaired immunity caused by factors such as vitamin D deficiency (11,16,30,31), dietary changes (16), and restricted social conditions (16,18), which cause an endogenous TB infection to reactivate from extrapulmonary or pulmonary sites. Also genetic factors (32), for example the presence of polymorphism of the NRAMP1 gene (33,34) may contribute to differences in the susceptibility to acquire extrapulmonary TB. Furthermore, M. tuberculosis strains circulating outside the Netherlands may be genetically different from those circulating in the Netherlands and cause more extrapulmonary TB.
A limitation in our study is the use of different methods of case finding, since immigrants from countries with a high prevalence are screened for pulmonary TB, but not for extrapulmonary TB (26). This circumstance could lead to a possible underestimation of extrapulmonary TB among immigrants, which would make the relationships found in this study even stronger. On the other hand, selection bias is possible when physicians disproportionately suspect and diagnose extrapulmonary TB among certain groups of patients such as non-Western patients. By including 97.0% of all reported new cases of TB in the analysis (13,258 of 13,673 new cases), we tried to minimize bias on inclusion in the study. Fewer patients with extrapulmonary TB had a positive culture than did patients with pulmonary TB (67.4% vs 71.5%). Thus patients with extrapulmonary TB may be misdiagnosed. However, a sensitivity analysis, which included only the culture-positive cases, did not change the conclusions of the logistic regression analysis. Also misdiagnosis of M. bovis as M. tuberculosis may have occurred in cases in which no culture result was available. Of the 114 M. bovis patients excluded from the analysis, all locations of TB were identified: 7 patients had pleural TB, 30 had lymphatic TB, 2 had meningeal TB, 3 had peritoneal TB, 8 had TB of bones and/or joints, 8 had genitourinary TB, 5 had miliary TB, 5 had other extrapulmonary TB, 42 had pulmonary TB, and 4 had extrapulmonary TB not specified.
It should be noted that nationality in the NTR is not always recorded based on passport; sometimes ethnic background is used. Furthermore, a difference in the definition of extrapulmonary TB used in different studies complicates mutual comparison.
Many studies suggest that HIV-induced immunosuppression is associated with extrapulmonary disease (7,35–39), especially with lymphatic and miliary TB (38,39). In the Netherlands, HIV testing is not a standard procedure for TB patients, which may have led to a possible selection bias in our study. A possible explanation for the absence of associations between HIV infection and most types of extrapulmonary TB in our study can be found in the introduction of the highly active antiretroviral therapy (HAART) in 1996 (40). HIV-infected patients treated with HAART will have less impaired immunity (39).
Diagnosing extrapulmonary TB can be difficult, so a high index of suspicion remains important. In immigrants from countries with highly endemic TB, a medical history, physical examination, basic laboratory tests, and chest radiograph can lead to a diagnosis. Since TB can occur in all organs, further examination depends on the possible site of infection.
In summary, our analysis showed that there is a proportional increase of extrapulmonary TB in the Netherlands. The growth of the number of inhabitants with a non-Western ethnic background in the Netherlands explains the proportional growth of extrapulmonary TB. Increased awareness among physicians about the changing clinical picture and up-to date knowledge about diagnosis of TB is warranted.
Dr te Beek is a medical officer at the Municipal Health Service in Schagen and at the Medical Centre for Handicapped Children "Parlan" in Den Helder, the Netherlands. She performed this study with the KNCV Tuberculosis Foundation as part of a specialization in public health at the Netherlands School of Public and Occupational Health, Amsterdam. Her research interests include infectious disease epidemiology.
Acknowledgment
We thank Dick van Soolingen, Petra de Haas, Nico Kalisvaart, and Frank Cobelens, for their help, and all the municipal health services in the Netherlands for their contribution to the Netherlands Tuberculosis Register.
References
- World Health Organization (WHO). Global tuberculosis control: surveillance, planning, financing. WHO report 2004. [cited 2006 Jul 18]. Available from http://www.who.int/tb/publications/global_report/2004/en/index.html
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. DOIPubMedGoogle Scholar
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. DOIPubMedGoogle Scholar
- Rieder HL. Epidemiology of tuberculosis in Europe. Eur Respir J Suppl. 1995;20:620s–32s.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Reported tuberculosis in the United States,1999, 2000, and 2001. [cited 2004 Oct 22]. Available at http://www.cdc.gov/nchstp/tb/surv/surv.htm
- Rieder HL, Snider DE Jr, Cauthen GM. Extrapulmonary tuberculosis in the United States. Am Rev Respir Dis. 1990;141:347–51.PubMedGoogle Scholar
- Gonzalez OY, Adams G, Teeter LD, Bui TT, Musser JM, Graviss EA. Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosis. Int J Tuberc Lung Dis. 2003;7:1178–85.PubMedGoogle Scholar
- Mehta JB, Dutt A, Harvill L, Mathews KM. Epidemiology of extrapulmonary tuberculosis. A comparative analysis with pre-AIDS era. Chest. 1991;99:1134–8. DOIPubMedGoogle Scholar
- Yang Z, Kong Y, Wilson F, Foxman B, Fowler AH, Marrs CF, Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004;38:199–205. DOIPubMedGoogle Scholar
- Noertjojo K, Tam CM, Chan SL, Chan-Yeung MM. Extra-pulmonary and pulmonary tuberculosis in Hong Kong. Int J Tuberc Lung Dis. 2002;6:879–86.PubMedGoogle Scholar
- Cowie RL, Sharpe JW. Extra-pulmonary tuberculosis: a high frequency in the absence of HIV infection. Int J Tuberc Lung Dis. 1997;1:159–62.PubMedGoogle Scholar
- Antony SJ, Harrell V, Christie JD, Adams HG, Rumley RL. Clinical differences between pulmonary and extrapulmonary tuberculosis: a 5-year retrospective study. J Natl Med Assoc. 1995;87:187–92.PubMedGoogle Scholar
- Chan-Yeung M, Noertjojo K, Chan SL, Tam CM. Sex differences in tuberculosis in Hong Kong. Int J Tuberc Lung Dis. 2002;6:11–8.PubMedGoogle Scholar
- Cowie RL, Sharpe JW. Tuberculosis among immigrants: interval from arrival in Canada to diagnosis. A 5-year study in southern Alberta. CMAJ. 1998;158:599–602.PubMedGoogle Scholar
- Moudgil H, Leitch AG. Extra-pulmonary tuberculosis in Lothian 1980–1989: ethnic status and delay from onset of symptoms to diagnosis. Respir Med. 1994;88:507–10. DOIPubMedGoogle Scholar
- Nisar M, Williams CS, Davies PD. Experience of tuberculosis in immigrants from South East Asia–implications for the imminent lease back of Hong Kong. Respir Med. 1991;85:219–22. DOIPubMedGoogle Scholar
- Ormerod LP, Nunn AJ, Byfield SP, Darbyshire JH. Patterns of tuberculosis in Indian and Pakistani/Bangladeshi patients: effects of age, date of first entry and ethnic group. Respir Med. 1991;85:275–80. DOIPubMedGoogle Scholar
- Kempainen R, Nelson K, Williams DN, Hedemark L. Mycobacterium tuberculosis disease in Somali immigrants in Minnesota. Chest. 2001;119:176–80. DOIPubMedGoogle Scholar
- Huebner RE, Castro KG. The changing face of tuberculosis. Annu Rev Med. 1995;46:47–55. DOIPubMedGoogle Scholar
- Barnes PF, Bloch AB, Davidson PT, Snider DE Jr. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–50. DOIPubMedGoogle Scholar
- Jones BE, Young SMM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292–7.PubMedGoogle Scholar
- Index tuberculosis 2000. The Hague: Royal Netherlands Tuberculosis Association; 2003.
- Statistics Netherlands. Allochtonen in Nederland [foreigners in the Netherlands]. Voorburg/Heerlen, the Netherlands: Statistics Netherlands; November 2003. p. 19–31. [cited 2006 Jul 18]. Available from http://www.cbs.nl/NR/rdonlyres/8AC144A6-5730-44B5-BDD2-D936F47C1F8D/0/2003b52p019art.pdf
- Wolleswinkel-van BJ, Nagelkerke NJ, Broekmans JF, Borgdorff MW. The impact of immigration on the elimination of tuberculosis in The Netherlands: a model based approach. Int J Tuberc Lung Dis. 2002;6:130–6.PubMedGoogle Scholar
- Verver S, van Soolingen D, Borgdorff MW. Effect of screening of immigrants on tuberculosis transmission. Int J Tuberc Lung Dis. 2002;6:121–9.PubMedGoogle Scholar
- van Burg JL, Verver S, Borgdorff MW. The epidemiology of tuberculosis among asylum seekers in The Netherlands: implications for screening. Int J Tuberc Lung Dis. 2003;7:139–44.PubMedGoogle Scholar
- Jutte PC, van Loenhout-Rooyackers JH, Borgdorff MW, van Horn JR. Increase of bone and joint tuberculosis in The Netherlands. J Bone Joint Surg Br. 2004;86:901–4. DOIPubMedGoogle Scholar
- Borgdorff MW, van der Werf M, de Haas PEW, Kremer K, van Soolingen D. Trend of tuberculosis transmission in the Netherlands, 1995–2002: a molecular epidemiologic analysis. Emerg Infect Dis. 2005;11:597–602.PubMedGoogle Scholar
- Statistics Netherlands. [cited 2005 Jan 12]. Available from http://statline.cbs.nl/StatWeb
- Rook GA. The role of vitamin D in tuberculosis. Am Rev Respir Dis. 1988;138:768–70. DOIPubMedGoogle Scholar
- Stead WW. Genetics and resistance to tuberculosis. Could resistance be enhanced by genetic engineering? Ann Intern Med. 1992;116:937–41.PubMedGoogle Scholar
- Kim JH, Lee SY, Lee SH, Sin C, Shim JJ, In KH, NRAMP1 genetic polymorphisms as a risk factor of tuberculous pleurisy. Int J Tuberc Lung Dis. 2003;7:370–5.PubMedGoogle Scholar
- Ma X, Dou S, Wright JA, Reich RA, Teeter LD, El Sahly HM, 5´ dinucleotide repeat polymorphism of NRAMP1 and susceptibility to tuberculosis among Caucasian patients in Houston, Texas. Int J Tuberc Lung Dis. 2002;6:818–23.PubMedGoogle Scholar
- Gilks CF, Brindle RJ, Otieno LS, Bhatt SM, Newnham RS, Simani PM, Extrapulmonary and disseminated tuberculosis in HIV-1-seropositive patients presenting to the acute medical services in Nairobi. AIDS. 1990;4:981–5. DOIPubMedGoogle Scholar
- Slutsker L, Castro KG, Ward JW, Dooley SW. Epidemiology of extrapulmonary tuberculosis among persons with AIDS in the United States. Clin Infect Dis. 1993;16:513–8. DOIPubMedGoogle Scholar
- Narain JP, Raviglione MC, Kochi A. HIV-associated tuberculosis in developing countries: epidemiology and strategies for prevention. Tuber Lung Dis. 1992;73:311–21. DOIPubMedGoogle Scholar
- Barnes PF, Bloch AB, Davidson PT, Snider DE Jr. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–50. DOIPubMedGoogle Scholar
- Aaron L, Saadoun D, Calatroni I, Launay O, Memain N, Vincent V, Tuberculosis in HIV-infected patients: a comprehensive review. Clin Microbiol Infect. 2004;10:388–98. DOIPubMedGoogle Scholar
- Smit C, Geskus R, Uitenbroek D, Mulder D, Van Den Hoek A, Coutinho RA, Declining AIDS mortality in Amsterdam: contributions of declining HIV incidence and effective therapy. Epidemiology. 2004;15:536–42. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 12, Number 9—September 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Marieke J. van der Werf, KNCV Tuberculosis Foundation, PO Box 146, 2501 CC, The Hague, the Netherlands
Top