Volume 13, Number 11—November 2007
Research
Distribution of Eosinophilic Meningitis Cases Attributable to Angiostrongylus cantonensis, Hawaii
Abstract
During November 2004–January 2005, 5 cases of eosinophilic meningitis (EM) attributable to Angiostrongylus cantonensis infection were reported in Hawaii. To determine if this temporal clustering reflected an increased incidence, we ascertained EM and A. cantonensis cases by systematic review of statewide laboratory and medical records for January 2001–February 2005 and generalized the data to population estimates. We identified 83 EM cases; 24 (29%) were attributed to A. cantonensis infection, which was included in the discharge diagnoses for only 2 cases. Comparison of A. cantonensis infection incidence rates (per 100,000 person-years) for the baseline (January 2001–October 2004) and cluster (November 2004–February 2005) periods showed statistically significant increases for the state as a whole (0.3 vs. 2.1), the Big Island of Hawaii (1.1 vs. 7.4), and Maui County (0.4 vs. 4.3). These findings underscore the need to consider the diagnosis of A. cantonensis infection, especially in the state of Hawaii.
Eosinophilic meningitis (EM) is a rare clinical entity characterized by meningeal inflammation and eosinophilic pleocytosis in the cerebrospinal fluid (CSF) (1–7). Among the infectious causes of EM, Angiostrongylus cantonensis is the most common worldwide. A. cantonensis, the rat lungworm, was first described in rats in 1935, in Canton, China. The parasite was first postulated to cause human infection in a fatal case in 1944 in Taiwan and was confirmed to be pathogenic for humans through investigations in the early 1960s in Hawaii (8–12).
Most of the described cases of symptomatic A. cantonensis infection (neurologic angiostrongyliasis) have occurred in regions of Asia and the Pacific Rim (e.g., Taiwan, Thailand, and the Hawaiian and other Pacific Islands) (4–19). However, widespread geographic dispersal of A. cantonensis is ongoing, facilitated primarily by infected shipborne rats and the diversity of potential intermediate hosts (9,20–27). Intercontinental movement of rodent definitive hosts and accidental human hosts translates into the need for worldwide awareness of the association between EM and A. cantonensis infection.
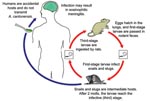
Humans become infected by ingesting intermediate hosts, such as snails and slugs, or transport/paratenic hosts, such as freshwater crustaceans, that contain viable third-stage larvae (Figure 1). These larvae can migrate to the central nervous system (CNS) and cause EM (6–34). The exposure often is unrecognized and presumptive, such as through ingestion of contaminated produce. The incubation period averages ≈1–3 weeks but has ranged from 1 day to >6 weeks (5–7,16–20,24,27,32–35). Common clinical manifestations include headache, meningismus, and hyperesthesia, which usually resolve spontaneously with supportive care; severe cases can be associated with sequelae (e.g., paralysis and blindness) and death (5,8,11,12,14–19,28,31,33–38). The utility of anthelminthic and corticosteroid therapy remains controversial and may vary among A. cantonensis–endemic areas (3,7,16–19,24,27–38).
Typically, symptomatic infection is presumptively diagnosed on the basis of epidemiologic and clinical criteria (4,5,7,13), as was done in this investigation. Parasitologic confirmation, by detection of larvae or young adult worms in the CSF, is unusual, albeit slightly more common in young children (particularly in Taiwan) (5,7,13–19,32). Investigational immunoassays for detection of antibodies to A. cantonensis antigens have not been sufficiently characterized or validated to be useful for distinguishing infected and uninfected persons, particularly in epidemiologic investigations (3,5,27).
During November 2004–January 2005, 1 parasitologically confirmed and 4 presumptive cases of A. cantonensis infection were reported to the Hawaii State Department of Health. The 5 cases included 3 from the Big Island of Hawaii and 2 from Oahu; 1 Oahu case was in a visitor to Hawaii whose lumbar puncture (LP) was performed elsewhere. Recognition of these 5 index cases prompted multifaceted investigations of epidemiologic, clinical, and environmental aspects of EM/A. cantonensis infection in Hawaii.
To assess whether the unusual temporal clustering of case reports reflected an increased incidence of EM/A. cantonensis infection, we ascertained cases through comprehensive review of statewide laboratory and medical records. Although investigations of EM/A. cantonensis infection in various Hawaiian Islands have been described since the 1960s (4–6,9–13,20,30,35), to our knowledge, this is the first study to systematically ascertain cases and determine regional incidence rates in this manner.
Ascertainment and Classification of Cases
Our primary means for ascertaining potential cases of EM and A. cantonensis infection was a retrospective review of CSF data provided by clinical laboratories in Hawaii for LPs performed during the study period (January 2001–February 2005). In March 2005, we obtained CSF data for 22 of 24 acute-care hospitals, which encompassed ≈93% of the state’s hospital beds; for 1 of the 22 facilities (≈7% of beds), data were unavailable for January 2001–December 2002. The total numbers of patients and LPs during the study period were unavailable (e.g., some laboratories provided CSF data only if particular criteria were met). In January 2005, 1 case of EM/A. cantonensis infection in a visitor to Hawaii whose LP was performed elsewhere was ascertained by passive physician reporting to the Hawaii State Department of Health and the Centers for Disease Control and Prevention (CDC); this case was 1 of the 5 index cases that prompted the investigation.
Our case definitions for EM and A. cantonensis infection are provided in Table 1. If the inclusionary criteria for EM were met, we reviewed the patient’s medical record to obtain additional information regarding the EM and to categorize cases of EM by known or likely cause (e.g., A. cantonensis infection). The information collected during chart review included basic demographic data, pertinent dates (e.g., birth, hospitalization, travel, symptom onset, and LP), medical history, medications, clinical manifestations, additional laboratory and radiologic results, and discharge diagnoses. Because the primary focus of the study was A. cantonensis infection, if, at the time of the LP, the patient had intracranial hardware (i.e., a well-established cause of EM) or was <2 months of age (i.e., angiostrongyliasis was epidemiologically unlikely), we collected only demographic data and discharge diagnoses.
We attributed cases of EM to A. cantonensis infection only if this diagnosis was epidemiologically and clinically plausible and no other possible cause of EM was identified. Examples of possible alternative causes included CNS infection with other microbes, reaction to foreign material in the CNS (e.g., intracranial hardware or myelography dye), medications (e.g., intrathecal vancomycin or gentamicin), neoplasms, multiple sclerosis, and neurosarcoidosis (1–7). The study neurologist (J.J.S.) facilitated final selection and classification of cases of EM and A. cantonensis infection by reviewing the available case data and ensuring that the inclusionary and exclusionary criteria were applied consistently and objectively.
Statistical Analysis and Human Subjects Protection
Data entry was performed with Epi Info version 2002 (CDC, Atlanta, GA, USA), and data analyses were conducted with SAS version 9.1 (SAS Institute, Cary, NC, USA). Two-tailed p values were calculated by using the Fisher exact test for binary variables and the Wilcoxon test for continuous variables. Linear and quadratic regression models were evaluated to assess whether eosinophilic pleocytosis varied with time (i.e., the interval from symptom onset to LP). We calculated incidence rates by generalizing hospital-based frequency data to the population at large for various periods and counties in Hawaii using the US Census Bureau’s annual population estimates for 2001–2004 (the estimate for 2004 also was used for January and February 2005) (39). We used Poisson regression analyses to compare county-specific annual rates. We defined the 46-month period of January 2001–October 2004 as the baseline period and the 4-month period of November 2004–February 2005 as the cluster period. CDC’s policies with regard to human study participants were followed in this investigation.
We identified 83 cases of EM for the 50-month study period (January 2001–February 2005); <1% of the patients whose CSF data were reviewed fulfilled the case criteria (Table 1). The 83 cases included 70 (84%) during the 46-month baseline period (17–21 cases per year) and 13 (16%) during the 4-month cluster period. We attributed 24 (29%) of the 83 EM cases to A. cantonensis infection and 59 (71%) to other causes (Table 2). Thirty-five of these 59 cases (42% of 83) were in persons with intracranial hardware, and 9 (11% of 83) were in persons without intracranial hardware who had documented bacterial (n = 5) or viral (n = 4) meningoencephalitis.
The 24 cases of EM attributed to A. cantonensis infection included 1 parasitologically confirmed case in an 11-month-old child and 23 clinically defined cases (Table 2). EM was noted in the discharge diagnoses for 11 case-patients (46%). A. cantonensis infection, as well as EM, was listed for only 2 cases: the parasitologically confirmed case and 1 other case in January 2005. The 24 case-patients had a median age of 31 years (range 11 months–45 years), and 13 (54%) were male. Of the 13 patients for whom race/ethnicity data were available, 6 (46%) were Caucasian, 3 (23%) Filipino, 3 (23%) Hawaiian/part-Hawaiian, and 1 (8%) Samoan.
For the 22 case-patients with known symptom onset dates, the median interval from onset to LP was 3 days (range 0–48 days); the 2 longest intervals were 14 days (2 patients) and 48 days (1 patient). When a linear regression model was applied to data for the intervals <14 days, the longer the interval (between symptom onset and LP), the higher the CSF eosinophil percentage and absolute eosinophil count (p = 0.001 and 0.005, respectively). Compared with patients with other causes of EM, A. cantonensis case-patients had significantly higher CSF leukocyte counts (median 573/mm3 vs. 304/mm3, p = 0.03) and absolute eosinophil counts (median 120/mm3 vs. 14/mm3, p<0.001); they also tended to have higher eosinophil percentages (median 15.0% vs. 12.0%), but the difference was not statistically significant (p = 0.08).
The temporal distribution of the 24 cases included 15 (63%) during the baseline period (3–5 cases per year) and 9 (38%) during the cluster period (Figure 2). The mean number of A. cantonensis cases per month increased from 0.3 in the baseline period to 2.3 in the cluster period, whereas the mean monthly rates for cases of EM with other causes were essentially unchanged (1.2 and 1.0, respectively). Thus, the proportion of EM cases attributed to A. cantonensis increased from 21% (15/70) for the baseline period to 69% (9/13) for the cluster period. The A. cantonensis incidence rates for the state as a whole increased from 0.3 per 100,000 person-years in the baseline period to 2.1 in the cluster period (p<0.001) (Figure 2).
The geographic distribution of the 24 cases included 3 counties and 4 islands: Honolulu County (Oahu Island; n = 11 cases, including the case in the visitor), Hawaii County (Big Island of Hawaii; n = 9, including the parasitologically confirmed case), and Maui County (n = 4, including 3 cases associated with Maui Island and 1 with Lanai). Although the absolute number of cases was highest for Honolulu, the county-specific incidence rates (per 100,000 person-years) for the study period as a whole were higher for Hawaii (1.4) and Maui (0.7) than Honolulu (0.3) (Figure 3). The case-patients were significantly more likely to have been in Hawaii County than Honolulu County (risk ratio 4.6, 95% confidence interval 1.9–11.1); the comparison between Hawaii and Maui Counties was not significant (data not shown). The increases in annualized incidence rates (cases/100,000 person-years) from the baseline to the cluster periods were statistically significant for Hawaii County (1.1 vs. 7.4; p<0.001) and Maui County (0.4 vs. 4.3; p = 0.03) but not for Honolulu County (0.2 vs. 1.0; p = 0.07) (Figure 3).
This study was prompted by an unusual temporal clustering of 5 reported cases of EM/A. cantonensis infection from 2 Hawaiian Islands during November 2004–January 2005. Our primary goal was to assess whether these voluntarily reported cases reflected an increased incidence. To accomplish this, we used a laboratory- and hospital-based approach to ascertain symptomatic cases of EM and A. cantonensis infection. To our knowledge, this is the first study to systematically determine incidence rates of EM and A. cantonensis infection for the entire state of Hawaii or any angiostrongyliasis-endemic area. We determined that the incidence of angiostrongyliasis was higher during the cluster period (November 2004–February 2005) than the baseline period (January 2001–October 2004). The overall findings of our study support conclusions specific for Hawaii but also highlight general principles about EM and A. cantonensis infection. In addition, our study may serve as a useful model in other settings. Surveillance of regional laboratory data, coupled with investigation of medical records of case-patients, may help identify temporal and geographic trends for angiostrongyliasis or other diseases.
Our data underscore that EM is an uncommon entity: <1% of the patients whose CSF data were reviewed fulfilled the laboratory criteria for EM. This diagnosis is commonly missed or dismissed, but the presence of eosinophilic pleocytosis is abnormal and should prompt consideration of both infectious and noninfectious causes. In our study, intracranial hardware was the most frequently identified cause of EM (42% of 83 cases). Although the presence of hardware or other foreign material in the CNS is a well-established cause of EM, the possibility of associated bacterial infection should be considered (2,6). In our study, EM also was associated with confirmed cases of bacterial and viral meningoencephalitis, as well as idiopathic cases (no microbial etiology identified) in infants evaluated because of fever or failure to thrive.
We found that a substantial proportion of the EM cases in Hawaii were attributable to A. cantonensis infection (29%) and that the proportion was 3-fold higher during the cluster than during the baseline period. This rate increase was particularly notable in Hawaii and Maui Counties. Despite the fact that 23 of the 24 cases were clinically defined, the likelihood of misclassification was low. By definition, none of the case-patients had another possible cause of EM identified. In most angiostrongyliasis-endemic areas, parasitologic confirmation is unusual, and a presumptive diagnosis is typical. Furthermore, Hawaii is hyperenzootic for infection with A. cantonensis but not Gnathostoma spinigerum or Baylisascaris procyonis, 2 other parasites commonly associated with EM. Our confidence that the A. cantonensis cases were correctly classified as such is further increased by the findings of other components of our multifaceted investigations, which included comprehensive epidemiologic and clinical characterization of patients, with longitudinal evaluation of clinical status and sequelae (N. Hochberg, unpub. data).
One of the limitations of our laboratory/hospital-based study is the likelihood that we underestimated the numbers of cases of EM and A. cantonensis infection. By definition, we did not include persons who were asymptomatic, were not medically evaluated, did not have an LP, did not have CSF data that met specified criteria for EM (e.g., if the LP was performed early or late in the course of infection, few eosinophils might have been noted), or did not meet conservative epidemiologic and clinical criteria. In addition, cases of EM/angiostrongyliasis that were associated with exposures in Hawaii but were diagnosed elsewhere were not systematically ascertained. Cases diagnosed after the end of the study period (February 2005) were not included (specifically, 2 cases reported in March and April 2005 that were associated with Hawaii County). Their existence, however, lends even more credence to the temporal clustering of cases in late 2004–early 2005.
A second limitation is that we cannot exclude the possibility that the temporal increases in frequency of cases were artifactual (e.g., reflected heightened awareness of A. cantonensis infection or decreased thresholds for performing LPs). However, the investigation was prompted by clustering of 5 voluntary case reports during November 2004–January 2005, when EM and A. cantonensis infection were not reportable conditions, and included a parasitologically confirmed case. In addition, for patients who accessed healthcare and had an LP, our methods for case ascertainment were not dependent upon clinicians considering or listing EM or A. cantonensis infection in discharge diagnoses. Our methods were systematic, statewide, and unbiased.
We recognize the limitations and the utility of the incidence data. We calculated incidence rates by generalizing relatively small numbers of cases to the population estimates for particular periods in the state and the pertinent counties. Adjusting the frequency data for the sizes of populations and the durations of periods facilitated comparisons between counties, periods, and causes of EM. The cases of EM not attributed to A. cantonensis served as a useful internal control for the conclusion that the incidence of angiostrongyliasis increased: the incidence of A. cantonensis infection was significantly higher during the cluster period, whereas the incidence of the other EM cases did not increase.
In conclusion, we demonstrated the utility of a comprehensive, laboratory/hospital-based approach for statewide surveillance of EM and A. cantonensis infection in Hawaii. We found a cluster of angiostrongyliasis cases between November 2004 through February 2005 primarily centered in Hawaii and Maui Counties. Furthermore, EM and A. cantonensis infection were often not included in the discharge diagnoses for the case-patients. Our study therefore underscores the need to educate clinicians in Hawaii and elsewhere about EM and its causes, most notably A. cantonensis infection, a potentially severe but preventable infection. Improved detection and reporting may facilitate recognition of clusters of cases and prompt investigations that yield valuable insights about the epidemiologic and clinical characteristics of A. cantonensis infection.
Dr Hochberg is an infectious disease fellow at Emory University. She is also a guest researcher with the Division of Parasitic Diseases at CDC, where she was an Epidemic Intelligence Service Officer at the time of this study. Her research currently focuses on the epidemiology of parasitic diseases.
Acknowledgments
We thank Eric Brown, Myra Ching-Lee, Rebecca L. Hall, Michele C. Hlavsa, Rob Hollingsworth, Jeffrey L. Jones, Norman O’Connor, James J. Sullivan, Chester Wakida, and John Williamson for invaluable assistance in various aspects of the investigations.
Financial support for this research came from CDC.
References
- Bosch I, Oehmichen M. Eosinophilic granulocytes in cerebrospinal fluid: analysis of 94 cerebrospinal fluid specimens and review of the literature. J Neurol. 1978;219:93–105. DOIPubMedGoogle Scholar
- Slom T, Johnson S. Eosinophilic meningitis. Curr Infect Dis Rep. 2003;5:322–8. DOIPubMedGoogle Scholar
- Kuberski T. Eosinophils in cerebrospinal fluid: criteria for eosinophilic meningitis. Hawaii Med J. 1981;40:97–8.PubMedGoogle Scholar
- Kuberski T, Wallace GD. Clinical manifestations of eosinophilic meningitis due to Angiostrongylus cantonensis. Neurology. 1979;29:1566–70.PubMedGoogle Scholar
- Hughes PA, Magnet AD, Fishbain JT. Eosinophilic meningitis: a case series report and review of the literature. Mil Med. 2003;168:817–21.PubMedGoogle Scholar
- Tangwanicharoen T, Viriyavejakul P, Punpoowong B, Wilairatana P, Kaewkungwal J, Pongponratn E, Cerebrospinal fluid analysis in eosinophilic meningoencephalitis. Southeast Asian J Trop Med Public Health. 2001;32:751–9.PubMedGoogle Scholar
- Beaver PC, Rosen L. Memorandum on the first report of Angiostrongylus in man, by Nomura and Lin, 1945. Am J Trop Med Hyg. 1964;13:589–90.PubMedGoogle Scholar
- Alicata JE. The discovery of Angiostrongylus cantonensis as a cause of human eosinophilic meningitis. Parasitol Today. 1991;7:151–3. DOIPubMedGoogle Scholar
- Horio SR, Alicata JE. Parasitic meningo-encephalitis in Hawaii: a new parasitic disease of man. Hawaii Med J. 1961;21:139–40.PubMedGoogle Scholar
- Rosen L, Chappell R, Laqueur GL, Wallace GD, Weinstein PP. Eosinophilic meningoencephalitis caused by a metastrongylid lung-worm of rats. JAMA. 1962;179:620–4.PubMedGoogle Scholar
- Rosen L, Loison G, Laigret J, Wallace GD. Studies on eosinophilic meningitis. 3. Epidemiologic and clinical observations on Pacific Islands and the possible etiologic role of Angiostrongylus cantonensis. Am J Epidemiol. 1967;85:17–44.PubMedGoogle Scholar
- Kuberski T, Bart RD, Briley JM, Rosen L. Recovery of Angiostrongylus cantonensis from cerebrospinal fluid of a child with eosinophilic meningitis. J Clin Microbiol. 1979;9:629–31.PubMedGoogle Scholar
- Yii CY. Clinical observations on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. Am J Trop Med Hyg. 1976;25:233–49.PubMedGoogle Scholar
- Yii CY, Chen CY, Chen ER, Hsieh HC, Shih CC, Cross JH, Epidemiologic studies of eosinophilic meningitis in southern Taiwan. Am J Trop Med Hyg. 1975;24:447–54.PubMedGoogle Scholar
- Tsai TH, Liu YC, Wann SR, Lin WR, Lee SJ, Lin HH, An outbreak of meningitis caused by Angiostrongylus cantonensis in Kaohsiung. J Microbiol Immunol Infect. 2001;34:50–6.PubMedGoogle Scholar
- Tsai HC, Liu YC, Kunin CM, Lee SS, Chen YS, Lin HH, Eosinophilic meningitis caused by Angiostrongylus cantonensis: report of 17 cases. Am J Med. 2001;111:109–14. DOIPubMedGoogle Scholar
- Punyagupta S, Bunnag T, Juttijudata P, Rosen L. Eosinophilic meningitis in Thailand: epidemiologic studies of 484 typical cases and the etiologic role of Angiostrongylus cantonensis. Am J Trop Med Hyg. 1970;19:950–8.PubMedGoogle Scholar
- Punyagupta S, Juttijudata P, Bunnag T. Eosinophilic meningitis in Thailand: clinical studies of 484 typical cases probably caused by Angiostrongylus cantonensis. Am J Trop Med Hyg. 1975;24:921–31.PubMedGoogle Scholar
- Kliks MM, Palumbo NE. Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med. 1992;34:199–212. DOIPubMedGoogle Scholar
- Prociv P, Spratt DM, Carlisle MS. Neuro-angiostrongyliasis: unresolved issues. Int J Parasitol. 2000;30:1295–303. DOIPubMedGoogle Scholar
- Campbell BG, Little MD. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am J Trop Med Hyg. 1988;38:568–73.PubMedGoogle Scholar
- New D, Little MD, Cross J. Angiostrongylus cantonensis infection from eating raw snails [letter]. N Engl J Med. 1995;332:1105–6. DOIPubMedGoogle Scholar
- Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med. 2002;346:668–75. DOIPubMedGoogle Scholar
- Lindo JF, Waugh C, Hall J, Cunningham-Myrie C, Ashley D, Eberhard ML, Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis. 2002;8:324–6. DOIPubMedGoogle Scholar
- Waugh CA, Shafir S, Wise M, Robinson RD, Eberhard ML, Lindo JF. Human Angiostrongylus cantonensis, Jamaica [letter]. Emerg Infect Dis. 2005;11:1977–8.PubMedGoogle Scholar
- Chen XG, Li H, Lun ZR. Angiostrongyliasis, Mainland China [letter]. Emerg Infect Dis. 2005;11:1645–7.PubMedGoogle Scholar
- Bowden DK. Eosinophilic meningitis in the New Hebrides: two outbreaks and two deaths. Am J Trop Med Hyg. 1981;30:1141–3.PubMedGoogle Scholar
- Tsai HC, Liu YC, Kunin CM, Lai PH, Lee SS, Chen YS, Eosinophilic meningitis caused by Angiostrongylus cantonensis associated with eating raw snails: correlation of brain magnetic resonance imaging scans with clinical findings. Am J Trop Med Hyg. 2003;68:281–5.PubMedGoogle Scholar
- Marsh CM. Eosinophilic meningitis/angiostrongyliasis from eating aquaculture-raised snails: a case report. Hawaii Med J. 1998;57:652–4.PubMedGoogle Scholar
- Batmanian JJ, O’Neill JH. Eosinophilic meningoencephalitis with permanent neurological sequelae [letter]. Intern Med J. 2004;34:217–8. DOIPubMedGoogle Scholar
- Wan KS, Weng WC. Eosinophilic meningitis in a child raising snails as pets. Acta Trop. 2004;90:51–3. DOIPubMedGoogle Scholar
- Tsai HC, Lee SS, Huang CK, Yen CM, Chen ER, Liu YC. Outbreak of eosinophilic meningitis associated with drinking raw vegetable juice in southern Taiwan. Am J Trop Med Hyg. 2004;71:222–6.PubMedGoogle Scholar
- Kliks MM, Kroenke K, Hardman JM. Eosinophilic radiculomyeloencephalitis: an angiostrongyliasis outbreak in American Samoa related to ingestion of Achatina fulica snails. Am J Trop Med Hyg. 1982;31:1114–22.PubMedGoogle Scholar
- Koo J, Pien F, Kliks MM. Angiostrongylus (Parastrongylus) eosinophilic meningitis. Rev Infect Dis. 1988;10:1155–62.PubMedGoogle Scholar
- Chotmongkol V, Sawanyawisuth K, Thavornpitak Y. Corticosteroid treatment of eosinophilic meningitis. Clin Infect Dis. 2000;31:660–2. DOIPubMedGoogle Scholar
- Chotmongkol V, Sawadpanitch K, Sawanyawisuth K, Louhawilai S, Limpawattana P. Treatment of eosinophilic meningitis with a combination of prednisolone and mebendazole. Am J Trop Med Hyg. 2006;74:1122–4.PubMedGoogle Scholar
- Chotmongkol V, Sawanyawisuth K. Clinical manifestations and outcome of patients with severe eosinophilic meningoencephalitis presumably caused by Angiostrongylus cantonensis. Southeast Asian J Trop Med Public Health. 2002;33:231–4.PubMedGoogle Scholar
- US Census Bureau. GCT-T1. Population estimates. Geographic area: Hawaii. 2004 [cited 2005 Jul 14]. Available from http://www.census.gov
Figures
Tables
Cite This Article1Current affiliation: Emory University, Atlanta, Georgia, USA
2Current affiliation: Stanford University School of Medicine, Stanford, California, USA
Table of Contents – Volume 13, Number 11—November 2007
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Natasha S. Hochberg, Division of Infectious Diseases, Emory University, 69 Jesse Hill Junior Dr SE, Atlanta, GA 30303, USA;
Top