Volume 15, Number 3—March 2009
Dispatch
Evaluation of Commercially Available Anti–Dengue Virus Immunoglobulin M Tests
Figure 1
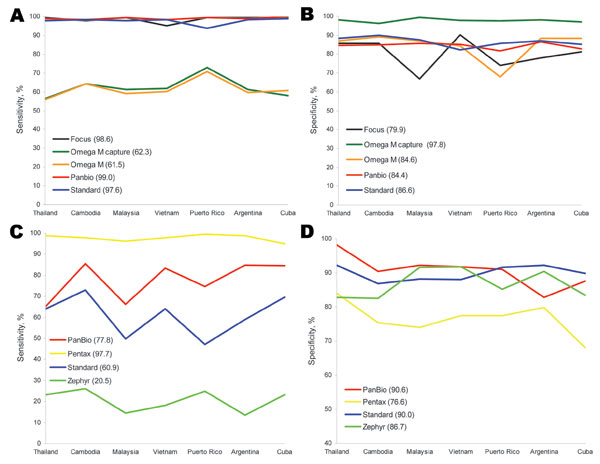
Figure 1. A) Sensitivity and B) specificity of 5 microplate ELISAs used at laboratories in 7 countries for detecting immunoglobulin (Ig) M against dengue virus compared with reference solid-phase IgM antibody-capture ELISAs developed by the Centers for Disease Control and Prevention (Atlanta, GA, USA) and the Armed Forces Research Institute of Medical Science (Bangkok, Thailand). Mean sensitivities and specificities for the 5 tests are shown in parentheses. C) Sensitivity and D) specificity of 4 rapid diagnostic tests used at laboratories in 7 countries for detecting IgM against dengue virus compared with solid-phase IgM antibody-capture ELISAs. Mean sensitivities and specificities for the 4 tests are shown in parentheses.
Page created: December 07, 2010
Page updated: December 07, 2010
Page reviewed: December 07, 2010
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.