Volume 16, Number 12—December 2010
Dispatch
Proportion of Deaths and Clinical Features in Bundibugyo Ebola Virus Infection, Uganda
Abstract
The first known Ebola hemorrhagic fever (EHF) outbreak caused by Bundibugyo Ebola virus occurred in Bundibugyo District, Uganda, in 2007. Fifty-six cases of EHF were laboratory confirmed. Although signs and symptoms were largely nonspecific and similar to those of EHF outbreaks caused by Zaire and Sudan Ebola viruses, proportion of deaths among those infected was lower (≈40%).
Ebola hemorrhagic fever (EHF) is a severe disease caused by several species of Ebolavirus (EBOV), in the family Filoviridae. Before 2007, four species of EBOV had been identified; 2 of these, Zaire ebolavirus and Sudan ebolavirus, have caused large human outbreaks in Africa, with proportion of deaths ≈80%–90% and 50%, respectively (1–5). Large outbreaks are associated with person-to-person transmission after the virus is introduced into humans from a zoonotic reservoir. Data suggest that this reservoir may be fruit bats (6,7). During outbreaks of EHF, the virus is commonly transmitted through direct contact with infected persons or their bodily fluids (8–11). The onset of EHF is associated with nonspecific signs and symptoms, including fever, myalgias, headache, abdominal pain, nausea, vomiting, and diarrhea; at later stages of disease, overt hemorrhage has been reported in ≈45% of cases (12).
Bundibugyo District is located in western Uganda, which borders the Democratic Republic of Congo. After reports of a mysterious illness in Bundibugyo District, the presence of a novel, fifth EBOV virus species, Bundibugyo ebolavirus (BEBOV), was identified in diagnostic samples submitted to the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA, in November 2007 (13). In response to detection of EBOV, an international outbreak response was initiated. In this report, we summarize findings of laboratory-confirmed cases of BEBOV infection.
Anecdotal reports suggested that human illness consistent with a viral hemorrhagic fever arose in Bundibugyo District as early as August 2007. After EHF was confirmed (13), isolation wards were established at 2 medical facilities in the district. Diagnostic samples from hospitalized patients with acute illness and community residents who had febrile illnesses and multiple additional signs, symptoms, or epidemiologic exposures suggestive of EHF, were routinely collected for EBOV testing. These signs and symptoms included headache, vomiting, diarrhea, abdominal pain, conjunctivitis, skin rash, muscle pain, fatigue, difficulty swallowing, difficulty breathing, hiccups, bleeding, unexplained death, or contact with a patient with suspected EHF. A laboratory-confirmed case of EHF was defined as illness in a person whose diagnostic samples were found positive for EBOV infection by any of the following tests: PCR (13), virus isolation, antigen detection, or immunoglobulin (Ig) M ELISA (14,15).
In addition, in a subset of surviving persons who had a history of illness consistent with EHF but no acute-phase blood samples available for testing, a convalescent-phase blood sample was collected for laboratory confirmation by IgG ELISA (14, 15). Laboratory testing was performed at the Uganda Viral Research Institute in Entebbe, and subsequent testing was performed on some samples at CDC, Atlanta. This analysis is limited to laboratory-confirmed EHF cases, although additional suspected cases were identified during the outbreak.
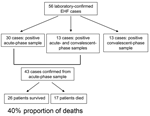
Fifty-six confirmed cases of EHF were identified; 43 of these were diagnosed on the basis of positive test results from acute-phase specimens (Figure). Twenty-six patients had a positive EBOV IgG titer in convalescent-phase serum, including 13 persons who had evidence of EBOV infection in acute-phase and convalescent-phase samples.
The proportion of deaths during this outbreak was calculated for case-patients confirmed on the basis of an acute-phase diagnostic sample (those who only had a convalescent-phase sample are by definition surviving case-patients, and represent a biased sample). Of the 43 cases confirmed from acute-phase samples, 17 deaths occurred, for a proportion of 40%. The mean age of those who died (42 years, range 20–70 years) was significantly higher than that of survivors (33 years, range 12–50 years; p = 0.0390). No gender bias was observed between survivors and those who died (Table).
Signs and symptoms were reported by patients on standardized surveillance case-report forms at the time of case identification, and in some instances, were examined further by chart review or follow-up interview. Common symptoms among patients with laboratory-confirmed cases included fever, fatigue, headache, nausea/vomiting, abdominal pain, muscle/joint pain, diarrhea, and anorexia/weight loss (Table). No difference in the proportion of those reporting signs and symptoms, or between those who survived and those who died was noted, except for difficulty swallowing. This symptom was more common among case-patients who died (though marginally significant, p = 0.0851). Bleeding of any type (bleeding from injection site, gums, eyes, nose, vagina; black or bloody stool; bloody vomitus; or hematuria) was reported among 54% of all patients with laboratory-confirmed cases.
As part of the standardized surveillance case-report form, patients were also asked whether they had had contact with a sick person during the 3 weeks before development of illness. A large portion of the laboratory-confirmed case-patients in this outbreak reported direct contact with a specific person, (case X), who died of a severe hemorrhagic febrile illness consistent with EHF (no diagnostic specimens were collected from this person) in November 2007. Using the date of last contact for those reporting contact with case X or reporting contact with another laboratory-confirmed case-patient to the date of symptom onset, we calculated an average incubation period of 6.3 days among laboratory-confirmed EHF case-patients (n = 24). No significant difference was noted in the incubation period between survivors (5.7 days) and those who died (7.4 days).
The 2007 outbreak of EHF in Bundibugyo District, Uganda, was caused by a new EBOV species, Bundibugyo ebolavirus (13). Previous outbreaks of EHF have resulted in high proportion of deaths, ranging from 50% to 90% (1–5). The case fatality proportion of deaths among case-patients with EHF confirmed by acute diagnostic specimens in this outbreak was 40%, a lower percentage than for other species of EBOV that have caused human outbreaks in Africa. However, we cannot exclude the possibility that the lower proportion of deaths in this outbreak is an artifact of differences in the severity of laboratory-confirmed cases detected through outbreak surveillance or the quality of care received by hospitalized laboratory-confirmed EHF case-patients in Bundibugyo District. Nonetheless, sustained person-to-person transmission was sufficient to result in a sizeable outbreak, and death was clearly not uncommon. Thus, BEBOV should be considered a pathogen of serious public health concern.
As with previously documented EHF outbreaks, older age appeared to be a risk factor for death (3). The incubation period of EHF was ≈1 week, and signs and symptoms were largely nonspecific; infections frequently involved fever, fatigue, headache, gastrointestinal involvement, and muscle and joint pain. The nonspecific nature of these signs and symptoms, which may mimic other tropical diseases, made diagnosis of EHF based on clinical characteristics alone particularly challenging and underscores the importance of laboratory-based diagnostics to confirm and monitor control and response efforts for EHF outbreaks. Notably, signs and symptoms described in this report are primarily based on information from patient surveillance case-report forms filled out at the time of triage, and they may not represent the full spectrum of illness experienced by all persons with EHF during this outbreak.
It is apparent that novel emerging infections continue to occur. The outbreak of EHF described in this report involved a previously unidentified EBOV species, with a proportion of deaths of 40%. BEBOV represents the fourth EBOV species–associated disease in humans, and the third species to cause large human outbreaks of EHF. Although proportion of deaths was lower than that documented in previous EHF outbreaks, BEBOV is a severe human pathogen with epidemic potential. These findings demonstrate the need for increased surveillance and diagnostic capabilities, as well as the capacity to respond quickly to emerging human infections.
Dr MacNeil is an epidemiologist with Viral Special Pathogens Branch, CDC. His research interests include the epidemiology and virology of zoonotic viral diseases.
Acknowledgments
We thank all persons who participated in the international outbreak response in Bundibugyo District, Uganda, during 2007–2008, including many persons from Médecins Sans Frontières, the World Health Organization, the African Field Epidemiology Network, the Uganda Ministry of Health, and multiple Epidemic Intelligence Service officers at CDC. We also acknowledge the medical staff in Bundibugyo District, Bundibugyo Hospital, and Kikyo Medical Center who provided care for patients.
Funding for this research was provided by CDC.
References
- Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–93.PubMedGoogle Scholar
- Khan AS, Tshioko FK, Heymann DL, Le Guenno B, Nabeth P, Kerstiens B, The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(Suppl 1):S76–86. DOIPubMedGoogle Scholar
- Sadek RF, Khan AS, Stevens G, Peters CJ, Ksiazek TG. Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995: determinants of survival. J Infect Dis. 1999;179(Suppl 1):S24–7. DOIPubMedGoogle Scholar
- Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team. Bull World Health Organ. 1978;56:247–70.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Outbreak of Ebola hemorrhagic fever Uganda, August 2000–January 2001. MMWR Morb Mortal Wkly Rep. 2001;50:73–7.PubMedGoogle Scholar
- Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–6. DOIPubMedGoogle Scholar
- Pourrut X, Delicat A, Rollin PE, Ksiazek TG, Gonzalez JP, Leroy EM. Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species. J Infect Dis. 2007;196(Suppl 2):S176–83. DOIPubMedGoogle Scholar
- Bausch DG, Towner JS, Dowell SF, Kaducu F, Lukwiya M, Sanchez A, Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(Suppl 2):S142–7. DOIPubMedGoogle Scholar
- Baron RC, McCormick JB, Zubeir OA. Ebola virus disease in southern Sudan: hospital dissemination and intrafamilial spread. Bull World Health Organ. 1983;61:997–1003.PubMedGoogle Scholar
- Dowell SF, Mukunu R, Ksiazek TG, Khan AS, Rollin PE, Peters CJ. Transmission of Ebola hemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidemies a Kikwit. J Infect Dis. 1999;179(Suppl 1):S87–91. DOIPubMedGoogle Scholar
- Roels TH, Bloom AS, Buffington J, Muhungu GL, Mac Kenzie WR, Khan AS, Ebola hemorrhagic fever, Kikwit, Democratic Republic of the Congo, 1995: risk factors for patients without a reported exposure. J Infect Dis. 1999;179(Suppl 1):S92–7. DOIPubMedGoogle Scholar
- Bwaka MA, Bonnet MJ, Calain P, Colebunders R, De Roo A, Guimard Y, Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–7. DOIPubMedGoogle Scholar
- Towner JS, Sealy TK, Khristova ML, Albarino CG, Conlan S, Reeder SA, Newly discovered Ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008;4:e1000212. DOIPubMedGoogle Scholar
- Ksiazek TG, Rollin PE, Williams AJ, Bressler DS, Martin ML, Swanepoel R, Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179(Suppl 1):S177–87. DOIPubMedGoogle Scholar
- Ksiazek TG, West CP, Rollin PE, Jahrling PB, Peters CJ. ELISA for the detection of antibodies to Ebola viruses. J Infect Dis. 1999;179(Suppl 1):S192–8. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 16, Number 12—December 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Adam MacNeil, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop G14, Atlanta, GA 30333, USA
Top