Volume 17, Number 11—November 2011
THEME ISSUE
CHOLERA IN HAITI
Dispatch
Drug-Resistance Mechanisms in Vibrio cholerae O1 Outbreak Strain, Haiti, 2010
Abstract
To increase understanding of drug-resistant Vibrio cholerae, we studied selected molecular mechanisms of antimicrobial drug resistance in the 2010 Haiti V. cholerae outbreak strain. Most resistance resulted from acquired genes located on an integrating conjugative element showing high homology to an integrating conjugative element identified in a V. cholerae isolate from India.
Vibrio cholerae is the bacterium that causes cholera, a disease characterized by acute watery diarrhea, vomiting, muscle cramps, and severe dehydration (1). The bacterium has many serogroups, but only toxin-producing serogroups O1 and O139 cause epidemic cholera. The primary treatment for cholera is rehydration with oral or intravenous fluids (2). For severe cases, antimicrobial agents may reduce the volume and duration of diarrhea (1,2). Tetracyclines (e.g., doxycycline), fluoroquinolones (e.g., ciprofloxacin), macrolides (e.g., erythromycin), and trimethoprim/sulfamethoxazole have commonly been used to treat cholera (2).
Antimicrobial drug resistance can undermine the success of antimicrobial therapy. Several reports have documented tetracycline- and fluoroquinolone-resistant V. cholerae, and multidrug resistance is increasing (3). Antimicrobial drug resistance in Vibrio spp. can develop through mutation or through acquisition of resistance genes on mobile genetic elements, such as plasmids, transposons, integrons, and integrating conjugative elements (ICEs). ICEs integrate and replicate with the host chromosome and can excise themselves and transfer between bacteria by conjugation (4). ICEs commonly carry several antimicrobial drug resistance genes and play a major role in the spread of antimicrobial drug resistance in V. cholerae (5). The first V. cholerae ICE described was in an O139 isolate in Madras, India, in 1992 and was named SXT after the resistance phenotype it conferred (trimethoprim/sulfamethoxazole) (6). Many O139 and O1 isolates have since acquired SXT or a closely related ICE (4,5).
We describe antimicrobial drug resistance mechanisms in the 2010 Haiti V. cholerae O1 outbreak strain. Most of the resistance is caused by acquired genes located on an ICE with high similarity to an ICE identified in a V. cholerae O1 isolated in India.
During October 2010–January 2011, a total of 122 clinical isolates of laboratory-confirmed V. cholerae O1 were recovered by the National Public Health Laboratory in Haiti and submitted to the Centers for Disease Control and Prevention (CDC; Atlanta, GA, USA) for characterization. Disk-diffusion antimicrobial drug susceptibility testing was performed at the National Public Health Laboratory and CDC. MICs were determined by broth microdilution at CDC by using Sensititer plates (CAMPY and CMV1AGNF; Trek Diagnostics, Cleveland, OH, USA) according to the manufacturer’s instructions with the following modifications: Mueller-Hinton broth without blood was used on the CAMPY plate, and for both plates, a final inoculum concentration of 5 × 104 to 5 × 105 CFU/mL was targeted. Escherichia coli American Type Culture Collection (ATCC; Manassas, VA, USA) 25922, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, and Pseudomonas aeruginosa ATCC 27853 were used for quality control testing. Where available, Clinical and Laboratory Standards Institute criteria specific for V. cholerae were used (7). For drugs lacking such criteria, manufacturers’ criteria, Clinical and Laboratory Standards Institute criteria for Enterobacteriaceae, or consensus breakpoints used by the National Antimicrobial Resistance Monitoring System were applied (8,9). Furazolidone was tested only by disk diffusion, and azithromycin was tested only by broth microdilution.
Results for all 122 outbreak isolates were similar. They showed susceptibility to azithromycin and tetracycline, reduced susceptibility to ciprofloxacin (MIC 0.25–1.0 mg/L), and resistance to furazolidone, nalidixic acid, sulfisoxazole, streptomycin, and trimethoprim/sulfamethoxazole.
With a common susceptibility pattern among all outbreak isolates, 1 isolate, 2010EL-1786 (deposited under ATCC BAA-2163), was chosen for molecular characterization. PCR was used to screen the isolate for the following resistance genes: strA, strB, sul1, sul2, dfrA1, dfrA10, and dfrA12 (10). In addition, the gyrA and parC genes were sequenced to identify quinolone resistance–determining region mutations. PCR was performed according to standard protocols by using the HotStarTaq PCR Master Mix (QIAGEN, Valencia, CA, USA). DNA sequencing was performed by using a 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
The isolate 2010EL-1786 contained strA/B, sul2, and dfrA1, which mediate resistance to streptomycin, sulfisoxazole/sulfamethoxazole, and trimethoprim, respectively. Nalidixic acid resistance and decreased susceptibility to ciprofloxacin were attributed to mutations in gyrA (Ser83Ile) and parC (Ser85Leu). The mechanism responsible for furazolidone resistance was not identified. Mutations in the nfsA and nfsB genes are associated with furazolidone resistance in E. coli, but inspection of the 2010EL-1786 sequence failed to identify these genes.
Location of the resistance genes was analyzed by whole-genome sequencing of 2010EL-1786. Single-end 454 pyrosequencing (GS FLX-Titanium; Roche Diagnostics, Indianapolis, IN, USA) reads and single-end 36-bp Illumina Solexa (GAIIe; Illumina, San Diego, CA, USA) reads were assembled de novo by using Newbler (Roche Diagnostics) and CLC Genomics Workbench (CLC bio, Cambridge, MA, USA) software. Sequence finishing was performed by using Sanger sequencing of fosmid clones (11).
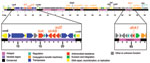
Whole-genome sequencing identified an ICE inserted in the prfC gene. This ICE, designated ICEVchHai1, was 97.9 kb and contained 95 open reading frames (Figure 1). All resistance genes identified were located on ICEVchHai1. In addition, floR, a chloramphenicol resistance gene, was detected. The strA, strB, sul2, and floR genes were part of an ≈17-kb fragment inserted into the rumB gene, whereas the dfrA1 gene was located ≈70 kb further downstream. Whole-genome sequencing also indicated a chloramphenicol acetyltransferase gene, catB9, that was not part of the ICE.
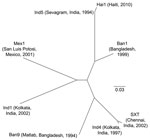
The genetic relatedness of ICEVchHai1 was assessed by comparison with 7 other ICE sequences (4). Sequence alignments were performed by using Progressive Mauve (http://asap.ahabs.wisc.edu/mauve/download.php) and visualized with PHYLIP version 3.69 (distributed by J. Felsenstein, Department of Genome Sciences, University of Washington, Seattle, WA, USA). ICEVchHai1 showed highest homology to ICEVchInd5, an ICE derived from a V. cholerae isolate from India (Figure 2). These ICEs differed by only 5 single-nucleotide polymorphisms.
In October 2010, an epidemic caused by toxigenic V. cholerae O1, serotype Ogawa, biotype El Tor strain, was reported from Haiti. We confirmed that the outbreak strain was multidrug resistant and displayed resistance to furazolidone, nalidixic acid, sulfisoxazole, streptomycin, and trimethoprim/sulfamethoxazole and decreased susceptibility to ciprofloxacin. Genetic mechanisms responsible for resistance to 5 of these drugs were identified. Sequencing also detected floR, a gene commonly associated with chloramphenicol resistance in Enterobacteriaceae (MICs >32 mg/L) (12). However, in this study, floR was not associated with resistance; isolates from Haiti displayed chloramphenicol MICs of 4–16 mg/L (7). Clinical non-Haiti isolates lacking the floR gene displayed MICs <1 mg/L. Why the floR gene did not confer resistance in V. cholerae remains to be investigated but might be because of lower expression levels. Sequencing also showed a chloramphenicol acetyltransferase gene, catB9. How this gene affects chloramphenicol MICs in V. cholerae remains to be determined.
Most of the acquired resistance genes were located on an ≈97-kbp ICE termed ICEVchHai1. The presence of an ICE in V. cholerae from Haiti was documented by Chin et al. in 2011 (13). ICEVchHai1 showed high homology to ICEVchInd5, an ICE first identified in a V. cholerae isolate from Sevagram, India, in 1994. Since then, ICEVchInd5 has persisted among clinical isolates in India; a recent study of O1 strains isolated in India during 1994–2005 confirmed that ICEVchInd5 was the only ICE that persisted during the study period (14).
Drug-resistant V. cholerae is a global health concern because resulting infections can be more severe and difficult to treat. Infections with drug-resistant V. cholerae can result in higher case-fatality rates, prolonged hospitalizations, more secondary infections, and increased health care costs. During an outbreak in Guinea-Bissau, case-fatality rates increased from 1% to 5.3% after the outbreak strain acquired multidrug resistance (15). To limit development and spread of antimicrobial drug resistance among V. cholerae, treatment with antimicrobial agents should be restricted to patients with severe dehydration or other conditions that truly warrant their use. Surveillance should continue for antimicrobial drug resistance among V. cholerae isolates from Haiti.
Dr Sjölund-Karlsson is a research microbiologist with the National Antimicrobial Resistance Surveillance Team at CDC. Her research interests include the characterization of antimicrobial drug–resistant bacteria, mechanisms of resistance, and studies of the biological cost of antimicrobial drug resistance.
References
- Sack DA, Lyke C, McLaughlin C, Suwanvanichkij V. Antimicrobial resistance in shigellosis, cholera, and campylobacteriosis. Geneva: World Health Organization; 2001 [cited 2011 Mar 23]. http://www.who.int/drugresistance/Antimicrobial_resistance_in_shigellosis_cholera_and_cam.pdf
- World Health Organization. Global Task Force on Cholera Control. First steps for managing an outbreak of acute diarrhoea. Geneva: The Organization; 2004 [cited 2011 Mar 23]. http://whqlibdoc.who.int/hq/2010/WHO_CDS_CSR_NCS_2003.7_Rev.2_eng.pdf
- Kitaoka M, Miyata ST, Unterweger D, Pukatzki S. Antibiotic resistance mechanisms of Vibrio cholerae. J Med Microbiol. 2011;60:397–407. DOIPubMedGoogle Scholar
- Wozniak RA, Fouts DE, Spagnoletti M, Colombo MM, Ceccarelli D, Garriss G, Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ICEs. PLoS Genet. 2009;5:e1000786. DOIPubMedGoogle Scholar
- Burrus V, Marrero J, Waldor MK. The current ICE age: biology and evolution of SXT-related integrating conjugative elements. Plasmid. 2006;55:173–83. DOIPubMedGoogle Scholar
- Waldor MK, Tschape H, Mekalanos JJ. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J Bacteriol. 1996;178:4157–65.PubMedGoogle Scholar
- Clinical and Laboratory Standards Institute. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline—second ed. M45–A2. Wayne (PA): The Institute; 2010 [cited 2011 Mar 23]. http://www.clsi.org/source/orders/free/m45-A2.pdf
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial suceptibility testing; twentieth informational supplement. Supplement M100–S20. Wayne (PA): The Institute; 2010 [cited 2011 Mar 23]. http://www.clsi.org/source/orders/free/m100-s20.pdf
- Centers for Disease Control and Prevention. National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): human isolates final report, 2008. Atlanta: The Centers; 2010.
- Chen S, Zhao S, White DG, Schroeder CM, Lu R, Yang H, Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Appl Environ Microbiol. 2004;70:1–7. DOIPubMedGoogle Scholar
- Reimer AR, Van Domselaar G, Stroika S, Walker M, Kent H, Tarr C, et al. Comparative genomics of Vibrio cholerae from Haiti, Asia, and Africa. Emerg Infect Dis. 2001;17: 2113–21.
- White DG, Hudson C, Maurer JJ, Ayers S, Zhao S, Lee MD, Characterization of chloramphenicol and florfenicol resistance in Escherichia coli associated with bovine diarrhea. J Clin Microbiol. 2000;38:4593–8.PubMedGoogle Scholar
- Chin CS, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364:33–42. DOIPubMedGoogle Scholar
- Ceccarelli D, Spagnoletti M, Bacciu D, Danin-Poleg Y, Mendiratta DK, Kashi Y, ICEVchInd5 is prevalent in epidemic Vibrio cholerae O1 El Tor strains isolated in India. Int J Med Microbiol. 2011;301:318–24. DOIPubMedGoogle Scholar
- Dalsgaard A, Forslund A, Petersen A, Brown DJ, Dias F, Monteiro S, Class 1 integron-borne, multiple-antibiotic resistance encoded by a 150-kilobase conjugative plasmid in epidemic Vibrio cholerae O1 strains isolated in Guinea-Bissau. J Clin Microbiol. 2000;38:3774–9.PubMedGoogle Scholar
Figures
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 17, Number 11—November 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Maria Sjölund-Karlsson, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop G29, Atlanta, GA 30333, USA
Top