Volume 18, Number 10—October 2012
Letter
Hand, Foot, and Mouth Disease Caused by Coxsackievirus A6
To the Editor: Coxsackievirus A6 (CVA6) is a human enterovirus associated with herpangina in infants. In the winter of 2012, we evaluated a cluster of 8 patients, 4 months–3 years of age, who were brought for treatment at Boston Children’s Hospital (Boston, MA, USA) with a variant of hand, foot, and mouth disease (HFMD) that has now been linked to CVA6 (Table). During this same period, the Boston Public Health Commission’s syndromic surveillance system detected a 3.3-fold increase in emergency department discharge diagnoses of HFMD. In the United States, HFMD typically occurs in the summer and early autumn and is characterized by a febrile enanthem of oral ulcers and macular or vesicular lesions on the palms and soles; the etiologic agents are most often CVA16 and enterovirus 71.
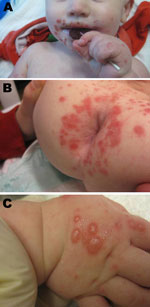
In contrast to the typical manifestation, the patients in the Boston cluster exhibited symptoms in late winter (Table) and had perioral (Figure, panel A) and perirectal (Figure, panel B) papules and vesicles on the dorsal aspects of the hands and feet (Figure, panel C). Patients experienced a prodrome lasting 1–3 days, consisting of fever (8 patients), upper respiratory tract symptoms (4 patients), and irritability (7 patients). This prodrome was followed by the development of a perioral papular rash (8 patients), which was often impetiginized with secondary crusting; a prominent papulovesicular rash on the dorsum of the hands and feet (6 patients); and a perirectal eruption (7 patients). Half of the patients had intraoral lesions. Fever abated in most of the patients within a day after onset of the exanthem. The rash resolved over 7–14 days with no residual scarring. Samples from the oropharynx, rectum, and vesicles from these patients were sent to the Centers for Disease Control and Prevention (Atlanta, GA, USA) for analysis. Reverse transcription PCR and sequencing by using primers specific for a portion of the viral protein 1 coding region identified CVA6 (1) (Table).
Outbreaks of HFMD caused by CVA6 have been described in Singapore, Finland, Taiwan, and most recently in Japan; most cases have occurred in the warmer months (2–6). Cases in the cluster described here are likely related to an emerging outbreak of CVA6-associated HFMD in the United States (7). The atypical seasonality of the outbreak, during the winter in Boston, could be related to the unusually mild temperatures in the winter of 2012.
Recent CVA6 outbreaks have been characterized by a febrile illness associated with an oral enanthem and lesions on the palms, soles, and buttocks. CVA6 infections in Taiwan during 2004–2009 were associated with HFMD in 13% of cases, with disease defined as oral ulcers on the tongue or buccal mucosa and vesicular rashes on the palms, soles, knees, or buttocks (2). In Singapore, where CVA6 accounted for 24% of HFMD cases, patients had oral lesions and <5 peripheral papules, placing them on a spectrum closer to the herpangina more typically observed in CVA6 infection (8).
The patients we report in this cluster most typically had perioral and perirectal papules in addition to vesicles on the dorsum of their hands. Two reports of CVA6-associated HFMD outbreaks describe cases that more closely resemble patients in the Boston outbreak. In a series from Finland in 2008, representative patients had both perioral lesions and vesicles on the dorsum of their hands (6). In a large series of patients with HFMD in Taiwan in 2010, patients with CVA6 had perioral lesions in addition to an enanthem (3).
Outbreaks of CVA6-associated HFMD in Finland, Taiwan, and Japan were associated with onychomadesis, with the loss of nails occurring 1–2 months after initial symptoms (3,4,6). The association between more typical HFMD and onychomadesis has additionally been described in the United States and Europe but without a link to specific serotype or with a small percentage of CVA6-associated cases (9). Cases from the Boston epidemic may fit into an emerging clinical phenotype of CVA6, and it will be interesting to see whether nail loss develops in those patients.
Given the numerous CVA6 outbreaks in multiple countries in 2008 and a US population that may be relatively naïve to this serotype, CVA6 is likely to spread throughout North America. Clinicians should be aware that, although standard precautions are routinely recommended for managing enteroviral infections in health care settings, contact precautions are indicated for children in diapers to control institutional outbreaks (10). In addition, the presence of perioral lesions and peripheral vesicles on the dorsum rather than palmar/plantar surface of the hands and feet represents a unique phenotype of HFMD that could be confused with herpes simplex or varicella-zoster virus infections. Because of the atypical presentation of CVA6-associated HFMD, clinical vigilance is needed to recognize emerging regional outbreaks. More detailed epidemiologic and genetic analyses will be required to characterize the role of CVA6 in US outbreaks of HFMD.
Acknowledgments
We thank Richard Rossi and Renee Roy for clinical sample preparation and processing and Kenneth McIntosh for critical review of this manuscript.
A.A.A. is supported by National Institutes of Health grant no. 5 K08 AI093676-02.
References
- Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–704. DOIPubMedGoogle Scholar
- Lo SH, Huang YC, Huang CG, Tsao KC, Li WC, Hsieh YC, Clinical and epidemiologic features of coxsackievirus A6 infection in children in northern Taiwan between 2004 and 2009. J Microbiol Immunol Infect. 2011;44:252–7. DOIPubMedGoogle Scholar
- Wei SH, Huang YP, Liu MC, Tsou TP, Lin HC, Lin TL, An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. DOIPubMedGoogle Scholar
- Fujimoto T, Iizuka S, Enomoto M, Abe K, Yamashita K, Hanaoka N, Hand, foot, and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012;18:337–9. DOIPubMedGoogle Scholar
- Blomqvist S, Klemola P, Kaijalainen S, Paananen A, Simonen ML, Vuorinen T, Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49–54. DOIPubMedGoogle Scholar
- Osterback R, Vuorinen T, Linna M, Susi P, Hyypia T, Waris M. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485–8. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Notes from the field: severe hand, foot, and mouth disease associated with coxsackievirus A6—Alabama, Connecticut, California, and Nevada, November 2011–February 2012. MMWR Morb Mortal Wkly Rep. 2012;61:213–4.PubMedGoogle Scholar
- Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:e1076–81. DOIPubMedGoogle Scholar
- Bracho MA, Gonzalez-Candelas F, Valero A, Cordoba J, Salazar A. Enterovirus co-infections and onychomadesis after hand, foot, and mouth disease, Spain, 2008. Emerg Infect Dis. 2011;17:2223–31. DOIPubMedGoogle Scholar
- Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(Suppl 2):S65–164. DOIPubMedGoogle Scholar
Figure
Table
Cite This Article1These authors contributed equally to this article.
Related Links
Table of Contents – Volume 18, Number 10—October 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Asim A. Ahmed, Children’s Hospital Boston–Infectious Diseases, 300 Longwood Ave, Boston, MA 02115, USA
Top