Volume 18, Number 3—March 2012
Dispatch
Hepatitis E Virus Infection in HIV-infected Persons
Abstract
To determine whether hepatitis E virus (HEV) is a cause of hepatitis among HIV-infected persons, we evaluated 1985–2009 data for US military beneficiaries. Evidence of acute or prior HEV infection was detected for 7 (4%) and 5 (3%) of 194 HIV-infected persons, respectively. HEV might be a cause of acute hepatitis among HIV-infected persons.
Among immunosuppressed persons in industrialized countries, hepatitis E virus (HEV) is a cause of sporadic acute viral hepatitis and chronic hepatitis (1,2). In the United States, liver test results are often abnormal for HIV-infected persons; however, few studies have evaluated whether HEV is a cause of hepatitis in this population (3).
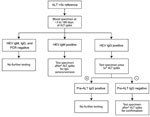
We retrospectively evaluated HIV-infected persons for whom alanine aminotransferase (ALT) levels had increased acutely (>5 × the upper limit of normal) during the HIV epidemic (1985–2009). Eligible participants were US military beneficiaries (persons entitled to receive care at a military treatment facility) for whom a stored serum specimen, collected from 3 days before ALT increase through 180 days after ALT increase, was available for HEV testing. A case of acute HEV infection was defined as a sample with HEV RNA and/or IgM against HEV or evidence of IgG seroconversion. All samples collected at the time of ALT increase were tested for IgM and IgG against HEV by using commercially available enzyme immunoassays (Diagnostics Systems, Nizhniy Novgorod, Russia) (4) and PCR for HEV RNA (5). The testing strategy is shown in Figure 1. All positive results were verified by retesting.
Statistical analyses included descriptive statistics presented as numbers (percentages) for categorical variables and medians (interquartile ranges [IQRs]) for continuous variables. The percentage of participants with HEV infection was defined as the number with an initial positive result for IgM or IgG against HEV divided by the total number of evaluable study participants. To compare characteristics among those with and without HEV infection, we used Fisher exact testing for categorical variables and rank-sum testing for continuous variables. A multivariate logistic regression model was used to identify factors associated with HEV infection. All analyses were performed by using Stata version 10.0 (StataCorp LP, College Station, TX, USA).
Among 4,410 HIV-infected persons, 458 (10%) had increased ALT levels at least 1 time during 32,468 person-years of follow-up. Among these, serum samples were available for HEV testing for 194 (42%) participants, among whom median age was 34 (IQR 30–40) years, 95% were male, and 42% were white (Table 1). The median ALT level was 440 (IQR 322–812) IU/mL. At the ALT spike, participants had been infected with HIV for a median duration of 5 (IQR 2–9) years; median CD4 cell count was 436 (IQR 239–627) cells/mm3, median plasma HIV RNA level was 13,581 (IQR 762–71,586) copies/mL, and 28% of participants were receiving antiretroviral therapy.
Samples for HEV testing were available at a median of 27 (IQR 0–104) days after the increase in ALT level. For 13 (6.7%) participants, IgM and/or IgG against HEV were present at the time closest to the ALT increase; antibody prevalence among those with elevated ALT levels did not increase during the HIV epidemic (χ2 0.76, p = 0.68). The 13 HIV-infected persons who were HEV seropositive (IgM or IgG at ALT spike) were similar to the 181 who were HEV seronegative in terms of demographics, military duty status, laboratory data, and overseas travel (Table 1). HEV-seropositive persons had higher plasma HIV RNA levels (4.7 vs. 4.1 log10 copies/mL, p = 0.04) and the association with lower CD4 cell counts was borderline (median 217 vs. 439 cells/mm3, p = 0.07). In the multivariate logistic regression model adjusted for age, plasma HIV RNA levels remained significantly associated with HEV seropositivity (odds ratio 1.96 per log10, 95% CI 1.04–3.71, p = 0.04).
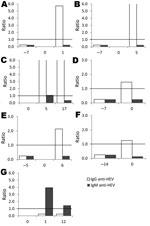
Additional testing was conducted for all 13 participants with IgM or IgG against HEV or with HEV RNA at the time of ALT increase (Figure 1). HEV RNA was detected in 1 participant who also seroconverted (IgG) at the time of ALT increase. According to samples collected at or near ALT spike and after ALT spike, 5 more participants seroconverted. One participant had IgM detectable in all 3 samples (at or near ALT spike, after ALT spike, and at follow-up); the participant did not seroconvert, and HEV RNA was not detectable in any sample. In total, 7 (3.6%, 95% CI 1.6%–7.6%) of the 194 HIV-infected persons had evidence of acute HEV infection at time of ALT spike (Figure 2). HEV was deemed not to be the cause of the ALT spike for 5 participants with evidence of prior HEV infection because IgG positivity preceded the ALT increase. For 1 participant, IgM was found in only 1 sample; all other samples were negative for IgM, IgG, and HEV RNA. Because of unconfirmed positive follow-up results, this participant was excluded from further analysis.
For the 7 participants with acute HEV infection (Table 2), HEV was not considered during the ALT increase, and HEV testing was not conducted as part of clinical care. No significant differences in clinical or laboratory characteristics were found among the 7 participants with acute HEV infection and those without evidence of HEV infection (data not shown). Chronic HEV infection did not develop in any participant.
HEV infection accounted for 4% of acute liver abnormalities among HIV-infected persons. Overall, HEV was detected in 6% of HIV-infected participants, similar to the 5%–21% reported earlier from the United States (1,6). Because study participation was limited to persons who had a sample available for HEV testing near the time of ALT increase, we might have missed cases of HEV infection. Overall, on the basis of our study and data from other industrialized countries (7,8), HEV is a cause of liver abnormalities in HIV-infected persons but does not seem to be more common in this population than in the general population.
HEV seropositivity did not increase over the course of the HIV epidemic. Despite increasing reports of HEV among HIV-infected persons and the general population (1,3,7–10), this increase is probably associated with increased recognition and testing. Recent studies in the United States and Europe have shown that HEV seroprevalence is stable or decreasing (1,11).
HEV infections among HIV-infected persons have been reported (3,7–9,12); however, whether this population is at increased risk for HEV infection remains uncertain. Recent studies from Europe suggest that HIV-infected persons or other immunocompromised persons are not at increased risk of acquiring HEV infections (7–9,12). Nonetheless, these groups are at higher risk for chronic HEV infection (2,7,13).
We propose that a diagnosis of HEV infection be considered for persons with viral-like hepatitis. Serologic test results may be negative despite ongoing HEV infection; hence, for HIV-infected persons (especially those with low CD4 cell counts), PCR testing for HEV RNA should be conducted (7). Because HEV infection may be fulminant in the presence of underlying liver disease (common among HIV-infected persons) (14) and may lead to chronic infection in immunosuppressed persons (2,13), testing should be considered for these persons as treatment options for HEV infection evolve. Moreover, chronic HEV infection may be averted by reducing the level of immunosuppression (15) and use of highly active antiretroviral therapy (3,8), but more data are needed to support these measures.
HEV infection is a newly defined cause of acute liver dysfunction among HIV-infected persons in the United States. HEV infections do not seem to preferentially occur among HIV-infected persons, suggesting that HIV itself may not be a risk factor for HEV acquisition. HEV infection should be considered among HIV-infected persons with liver abnormalities of unclear etiology.
Members of The Infectious Disease Clinical Research Program HIV Working Group are Susan Banks, Irma Barahona, Mary Bavaro, Helen Chun, Cathy Decker, Lynn Eberly, Conner Eggleston, Susan Fraser, Joshua Hartzell, Gunther Hsue, Arthur Johnson, Mark Kortepeter, Alan Lifson, Michelle Linfesty, Grace Macalino, Scott Merritt, Robert O’Connell, Jason Okulicz, Sheila Peel, Michael Polis, John Powers, Roseanne Ressner, Sybil Tasker, Edmund Tramont, Tyler Warkentien, Paige Waterman, Timothy Whitman, Glenn Wortmann, and Michael Zapor.
Acknowledgments
We thank Tracy Green-Montfort and Ngoc-Thao Le for their help with HEV testing and Chong-Gee Teo for his critical review of the manuscript.
Support for this work (IDCRP-000-21) was provided by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Inter-Agency Agreement Y1-AI-5072.
Dr Crum-Cianflone is an infectious disease staff physician at the Naval Medical Center San Diego, adjunct professor at San Diego State University, and a voluntary associate professor of medicine at the University of California San Diego. Her research has focused on complications and co-infections among HIV-infected persons.
References
- Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988–1994. J Infect Dis. 2009;200:48–56. DOIPubMedGoogle Scholar
- Kamar N, Selves J, Mansuy J, Ouezzani L, Péron JM, Guitard J, Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–7. DOIPubMedGoogle Scholar
- Curry JA, Adams N, Crum-Cianflone NF. Acute hepatitis E infection in an HIV-infected person in the United States. Ann Intern Med. 2009;150:226–7.PubMedGoogle Scholar
- Drobeniuc J, Meng J, Reuter G, Greene-Montfort T, Khudyakova N, Dimitrova Z, Serologic assays specific to immunoglobulin M antibodies against hepatitis E virus: pangenotypic evaluation of performances. Clin Infect Dis. 2010;51:e24–7. DOIPubMedGoogle Scholar
- Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. DOIPubMedGoogle Scholar
- Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–22. DOIPubMedGoogle Scholar
- Kenfak-Foguena A, Schöni-Affolter F, Bürgisser P, Witteck A, Darling KE, Kovari H, ; Data Center of the Swiss HIV Cohort Study, Lausanne, Switzerland. Hepatitis E virus seroprevalence and chronic infections in patients with HIV, Switzerland. Emerg Infect Dis. 2011;17:1074–8. DOIPubMedGoogle Scholar
- Keane F, Gompels M, Bendall R, Drayton R, Jennings L, Black J, Hepatitis E virus coinfection in patients with HIV infection. HIV Med. 2012;13:83–8.PubMedGoogle Scholar
- Renou C, Lafeuillade A, Cadranel JF, Pavio N, Pariente A, Allègre T, Hepatitis E virus in HIV-infected patients. AIDS. 2010;24:1493–9. DOIPubMedGoogle Scholar
- Colson P, Kaba M, Moreau J, Brouqui P. Hepatitis E in an HIV-infected patient. J Clin Virol. 2009;45:269–71. DOIPubMedGoogle Scholar
- Mansuy JM, Abravanel F, Miedouge M, Mengelle C, Merviel C, Dubois M, Acute hepatitis E in south-west France over a 5-year period. J Clin Virol. 2009;44:74–7. DOIPubMedGoogle Scholar
- Madejón A, Vispo E, Bottecchia M, Sánchez-Carrillo M, García-Samaniego J, Soriano V. Lack of hepatitis E virus infection in HIV patients with advanced immunodeficiency or idiopathic liver enzyme elevations. J Viral Hepat. 2009;16:895–6. DOIPubMedGoogle Scholar
- Dalton HR, Bendall R, Keane F, Tedder R, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361:1025–7. DOIPubMedGoogle Scholar
- Ramachandran J, Eapen CE, Kang G, Abraham P, Hubert DD, Kurian G, Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134–8. DOIPubMedGoogle Scholar
- Kamar N, Abravanel F, Selves J, Garrouste C, Esposito L, Lavayssière L, Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation. 2010;89:353–60. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1Members are listed at the end of this article.
Table of Contents – Volume 18, Number 3—March 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nancy F. Crum-Cianflone, c/o Clinical Investigation Department (KCA), Naval Medical Center San Diego, Suite 5, 34800 Bob Wilson Dr, San Diego, CA 92134-1005, USA
Top