Volume 19, Number 7—July 2013
Research
Quantifying Effect of Geographic Location on Epidemiology of Plasmodium vivax Malaria
Abstract
Recent autochthonous transmission of Plasmodium vivax malaria in previously malaria-free temperate regions has generated renewed interest in the epidemiology of this disease. Accurate estimates of the incubation period and time to relapse are required for effective malaria surveillance; however, this information is currently lacking. By using historical data from experimental human infections with diverse P. vivax strains, survival analysis models were used to obtain quantitative estimates of the incubation period and time to first relapse for P. vivax malaria in broad geographic regions. Results show that Eurasian strains from temperate regions have longer incubation periods, and Western Hemisphere strains from tropical and temperate regions have longer times to relapse compared with Eastern Hemisphere strains. The diversity in these estimates of key epidemiologic parameters for P. vivax supports the need for elucidating local epidemiology to inform clinical follow-up and to build an evidence base toward global elimination of malaria.
The malaria parasite Plasmodium vivax, which received limited research attention for a number of decades, has moved onto the global health agenda for 2 key reasons. First, compared with the P. falciparum parasite, which also causes malaria, the P. vivax parasite is more difficult to eliminate because it has a broader geographic range and, unlike P. falciparum, has dormant liver stages. Second, P. vivax malaria has reemerged in previously malaria-free temperate regions, including Greece, Corsica, the Korean Peninsula, central China, and Australia (1–5). Moreover, increasing evidence indicates that P. vivax infections can be severe and fatal (6,7).
A large body of epidemiologic and clinical data supports the existence of discrete strains within each Plasmodium species. Much of the reliable data are from patients who were infected in temperate climates (study centers in the United States, United Kingdom, and Europe), and the parasites were from various locales. Tropical vivax strains generally cause a larger number of closely spaced relapses, and temperate strains have generally evolved to cause a long incubation period, enabling the survival of the parasite as dormant hypnozoites during colder months (8,9). Malariologists have long recognized that the incubation period for malaria varies by strain and geographic latitude (10).
Recent studies of temperate P. vivax strains in South Korea support these observations and suggest that chemoprophylaxis of infected patients may contribute to long latency (11,12). However, these observational studies could not estimate the time from infection to relapse or relapse periodicity because exact infection times were unknown. In a related primaquine dosing study, the relapse rate for 3 vivax strains was compared, but relapse time was not examined (13).
Numerous P. vivax classification schemes have been suggested, including temperate/tropical, temperate/subtropical/temperate, and northern/southern/Chesson-type. These schemes were suggested on the basis of observed clinical characteristics, but quantitative data to support these distinctions are sparse (14–16). Recent molecular and entomologic data suggests that P. vivax may consist of 2 subspecies, 1 in the Old World/Eastern Hemisphere (P. vivax vivax) and the other in the Americas (P. vivax collins) (17); however, research with other isolates has not confirmed these results (18). These 2 strains/subspecies show remarkable differences in their infectivity to different Anopheles spp. mosquitoes; however, the effect of these differences on the epidemiology of malaria in humans has not been demonstrated. In addition, at least 2 other subspecies, P. vivax hibernans and P. vivax multinucleatum, produce disease that is similar to currently circulating strains from the Korean Peninsula (19,20).
A large body of historical data exists from the deliberate laboratory infection of 2 populations: 1) patients receiving malariotherapy (intentionally induced malaria) for neurosyphilis and related disorders; and 2) healthy prisoners who participated in malaria drug testing trials. We used the experimental infection data from these controlled settings to explore and quantify the relationship between P. vivax parasite origin and characteristics of malaria caused by these subpopulations.
Data Sources and Selection Criteria
Search Strategy
We performed a comprehensive literature search of MedLine and Google Scholar in English, searching for (“vivax” OR “benign tertian”) AND (“induced” OR “human” OR “experimental”). Limited searches were performed in Dutch, German, and French. The citations in these initial papers were examined, resulting in the identification of a large number of non-indexed papers.
Data Inclusion Criteria
We included data for experimentally infected persons who 1) were malaria naive before the experimental infection, 2) had defined inoculation dates (infection by mosquito only; persons with infections from sporozoite injection or blood transfer were excluded), 3) had received only well-documented (or explicit) treatment of symptoms, 4) were protected against reinfection, and 5) were infected with traceable strains that had defined origins. Studies that measured prepatent period (i.e., the time to first appearance of blood-stage parasites, seen by microscopy) were excluded. Data for patients with incubation periods >50 days were excluded from the analysis because only 7 such cases were found in well-defined studies. For the infection relapse study, we included data only from studies that had unambiguous follow-up periods. Available covariates were extracted from these studies, and the individual records were digitized by using Plot Digitizer (http://plotdigitizer.sourceforge.net), as needed, to create individual case-patient records.
Our incubation period analyses included data for 453 patients (infected with a total of 11 strains) from 19 studies (Table 1); data for 6 of the patients were excluded because of highly outlying covariate patterns (Technical Appendix). The first relapse analyses included data for 320 patients (infected with a total of 18 strains) from 15 studies (Table 1); data for 4 of the patients were excluded because of highly outlying covariate patterns (Technical Appendix). Details of the parasite strains are shown in Table 2. Age and sex were not recorded for most patients who had neurosyphilis. All prison volunteers were White men. Several sources had interval censoring; that is, infections were reported as occurring within 1 month or 16 weeks of the mosquito bite. To facilitate comparisons between studies that reported relapse times by integer weeks, relapse dates reported as exact days were analyzed as the next full week from infection. The final relapse event in these data occurred 65 weeks after initial infection; longer follow-up times were right-censored at 78 weeks from initial infection. Latitude of parasite isolation was determined from the site of isolation in the original reports and coded as a binary variable (dividing at ±23.5° South/North latitude) for determining tropical and temperate P. vivax parasites. In our analyses, New World strains are those from the Americas, and Old World strains are those from Eurasia, Africa, and the Pacific region, as suggested by Li et al. (17).
Statistical Analyses
Kaplan-Meir analysis was used to examine the unadjusted relationship between parasite origin and event times; differences were assessed by using a log-rank test. Multivariate models were used to overcome limitations of Kaplan-Meier analyses by allowing for adjustment for the effect of malariotherapy and by producing hazard ratios (HRs) to gauge the strength of association. The standard Cox proportional hazards model cannot provide confidence intervals for predicted survival times; therefore, the more complex, flexible parametric Royston-Parmar models were used to provide covariate-adjusted HRs and covariate-adjusted median survival times for parasite subpopulations (21). These models extend Cox methods by adding parameters that model the underlying hazard of a disease event, which allows for more comprehensive predictions. To provide estimates that are relevant to natural malaria infections in humans, the predicted survival times from this analysis were made for a population not receiving treatment for neurologic symptoms.
The incubation period and time-to-relapse models include the geographic region and neurologic treatment status as covariates for survival time; for the time-to-relapse models, geographic region was modeled as a time-varying covariate due to proportional hazard violations (the effect of regions is allowed to vary through follow-up time). Both models also incorporate adjustments for intragroup correlation caused by the clustering of effects among persons infected with the same parasite strain.
We used Stata 12.1 (StataCorp, College Station, TX, USA) to perform statistical analyses; all tests were 2-tailed. Detailed methods are in the online Technical Appendix.
Incubation Period
The Kaplan-Meier plot of 447 case-patients who were included in the incubation period study shows a wide separation of Old World/New World groups by tropical/temperate region (Figure 1, panel A) and latitude/Hemisphere (Figure 1, panel C). The separations are statistically discernible by tropical/temperate region (log-rank test for equality χ2 = 127.9, 1 df, p<0.0001) and by latitude/hemisphere (log-rank test for equality χ2 = 204.6, 3 df, p<0.0001).
The unadjusted median incubation period for malaria caused by the combined tropical strains was 12 days (95% CI 12–12); that for the combined temperate strains was 15 days (95% CI 14–16). When stratified by Old World/New World, the tropical strains remain essentially unchanged, but a large separation occurred in the temperate strains: median survival was 14 days (95% CI 14–15) for New World temperate strains and 20 days (95% CI 19–21) for Old World temperate strains.
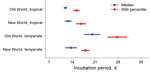
In the full multivariate model, after adjustment for neurologic treatment status, the median parametric survival estimate for the entire population was 13.6 days (95% CI 12.4–14.7) (Figure 1, panel E). The 95th percentile for the incubation period was 17.8 days (95% CI 16.6–18.9). Adjusted HRs differed between all regional categories, except for New World tropical/temperate categories, which did not achieve significance (p = 0.30). Predicted median and 95th percentile survival times are shown in Figure 2. Predicted median survival times were not different within the confidence intervals, except for Old World temperate strains (20.1 days [95% CI 17.8–22.5]); the 95th percentiles for temperate strains were significantly longer than those for tropical strains. HRs were 16.8 (95% CI 7.7–36.9) for the Old World, tropical region; 10.8 (95% CI 4.6–25.2) for the New World, tropical region; 7.3 (95% CI 3.8–14.0) for the New World, temperate region—all relative to the Old World, temperate region (reference). Persons infected with Old World tropical strains had a 16.8 (95% CI 7.7–36.9) times higher risk of clinical infection than did those with Old World temperate strains at each time point, leading to a shorter incubation period.
Time to First Malaria Relapse
In all studies, the time to first relapse was measured from the reported primary infection. We used Kaplan-Meir survival curves to compare relapse times for the 316 persons included in these analyses and found large, statistically significant differences by tropical/temperate regions (log rank test for equality χ2 = 56.2, 1 df, p<0.0001) (Figure 1, panel B) and by latitude/hemisphere (log rank test for equality χ2 = 198.9, 3 df, p<0.0001) (Figure 1, panel D). Figure 1, panel D, shows that infections caused by the 2 Old World categories show shorter times to relapse compared with infections from New World parasites. In the full multivariate model (adjusted for neurologic treatment status), we found a distinct separation between the hemispheres (Figure 1, panel F); for the total population, we estimated the median time to relapse was 29.2 weeks (95% CI 25.0–33.4) and the 95th percentile was 61.4 weeks (95% CI 35.7–87.1).
However, these aggregate values obscure substantial heterogeneity in time to relapse. Median relapse times for malaria caused by Old World parasites (tropical, 4.5 weeks [95% CI 3.6–5.4]; temperate, 8.5 weeks [95% CI 6.8–10.3]) were shorter than those for malaria caused by New World parasites (tropical, 27.5 weeks [95% CI 21.6–33.5]; temperate, 34.0 weeks [95% CI 32.0–36.0]). In addition, in both hemispheres, median relapse times for infections caused by tropical strains were shorter than those for infections caused by corresponding temperate strains, although this difference was not significant in the New World (Figure 3). The 95th percentile relapse times for the strain categories follow: Old World tropical, 9.5 weeks (95% CI 5.4–13.5); New World tropical, 40.3 weeks (95% CI 34.4–46.3); Old World temperate, 30.9 weeks (95% CI 19.9–41.9); and New World temperate, 97.7 weeks (95% CI 97.6–97.8). The HRs from the survival models (adjusted for neurologic treatment) follow: Old World tropical, 39.6 (95% CI 9.2–171.0; p<0.001); New World tropical, 0.93 (95% CI 0.36–2.41; p = 0.89); Old World temperate, 3.1 (95% CI 2.2–4.6; p<0.001)—all relative to New World temperate (reference).
Distribution of Relapses
The total number of relapses was compared by latitude and hemisphere (Technical Appendix). An interval of 48 weeks was chosen to ensure equivalent follow-up periods between the regions (1-way analysis of variance F-test, follow-up time by region [p = 0.37]). To test the equality of distributions, we use the Kolmogorov-Smirnov test and found significant differences between tropical and temperate strains (p<0.001) and Old World and New World strains (p = 0.001). These differences remained significant when stratified by zones of latitude: temperate strains, Old World versus New World (p = 0.001); and tropical strains, Old World versus New World (p<0.001).
Malariologists have made a large number of observations concerning the geography-related epidemiology of P. vivax malaria (22,23). Malariotherapy treatments initially used a range of local strains from the United Kingdom, the Netherlands, and the United States, but these were quickly replaced with the Madagascar strain and others, which exhibited shorter incubation periods and more reliably produced infections (24). Estimates for the incubation period of P. vivax malaria vary: mean of 13.9 days (SD 3.7); 14 ± 3 days after the mosquito bite; 12–17 days (mean 15); and 8–17 days (25–28). A modern quantitative analysis of data for malariotherapy with a single strain (Madagascar) provided estimates of ≈10.3–16.9 days for the prepatent period (reported, in the study, to be generally 3 days longer than the incubation period) (29). After adjusting for neurologic treatment status, the estimated median from the parametric models for the population in our study is 13.6 days (95% CI 12.5–14.7), and the incubation period 95th percentile was 17.8 days (95% CI 16.6–18.9). The minimal differences between the Kaplan-Meir estimates and those from the multivariate models for incubation period strongly support earlier opinions that malariotherapy data are applicable to natural infections (30).
A range of strain-specific observations has also been reported for malaria relapses (27). Compared with persons with malaria caused by temperate strains, persons with malaria caused by tropical strains relapse more and have shorter relapses intervals (17–45 days), and a higher proportion have >2 relapses (26). It has been estimated that malaria caused by tropical strains relapses every 3–4 weeks, whereas malaria caused by temperate strains has longer, more variable periods between relapses (31).
Our finding suggests that these prior estimates for relapse intervals were based primarily on infections with Old World tropical strains. Our time-to-relapse estimates for other regions are considerably longer than estimates from earlier studies, with the exception of a study from El Salvador, which reported a median relapse interval of 28 weeks (32). The arithmetic median interval for malaria caused by all tropical strains (including only exact, non–interval censored times) was 20.0 weeks (95% CI 11.9–28.1). Results of the unadjusted Kaplan-Meier and parametric models, adjusted for neurologic treatment status in the full dataset, also indicate much longer median times to relapse: 20 weeks (95% CI 9–26) and 10.5 weeks (95% CI 3.7–17.4), respectively. Persons infected with Old World temperate strains have 39 (95% CI 9.2–171.0) times higher risk of relapse at each time point relative to those infected with New World temperate strains. Figure 1, panels D and F, suggests that the interaction between neurologic treatment and infection with different parasite strains has a substantial effect on the course of relapse; therefore, unadjusted relapse times from malariotherapy studies should be interpreted with caution.
In the data we studied, the number of relapses recorded within 48 weeks (for equivalent follow-up) is consistent with prior estimates: median of 2.6 relapses (95% CI 1.9–3.3; range 0–9) and of 0.68 relapses (95% CI 0.55–0.80; range 0–6) for case-patients with malaria caused by tropical and temperate strains, respectively. The data also showed that 68.5% (95% CI 64.9%–71.8%) of case-patients had relapses; this finding is broadly consistent with the previous finding that ≈60% of untreated cases relapse (27).
The general agreement of the number of relapses and incubation period among the population we studied with prior estimates suggests that the patients in our study do not represent a population substantially different from those with naturally acquired infections. However, the aggregate cohort values obscure large regional differences in epidemiology.
The effect of the geographic location of malaria parasites on the epidemiology of malaria has been long recognized: conspicuous differences in incubation period and disease latency have been broadly correlated with climatic zones (27). However, there have also been persistent difficulties in classifying these patterns; 2 different types of temperate strains (North American [St. Elizabeth] and European [Netherlands]) as well as tropical strains have been suggested (14). Inconsistencies have also been noted. For example, the tropical P. vivax strains in Central America show anomalous temperate zone epidemiology, leading to a suggestion that temperature alone might be an insufficient predictor of regional epidemiology (33). These conflicting observations are consistent with the results from this study (Figure 1, panel F), in which the most noticeable feature is that both categories of New World parasites caused malaria with substantially longer times to relapse, compared with malaria caused by Old World parasites.
Our findings suggest that the epidemiology of P. vivax infection has been occluded by inherent differences between parasite subpopulations and that hemisphere and latitude are strong drivers of the clinical manifestations and epidemiology of malaria. These findings strongly suggest that current paradigms for P. vivax clinical follow-up and surveillance may be based on erroneous assumptions. Malaria should not be discounted as a diagnosis even in the presence of long incubation periods, and the geographic origin of the parasite has critical effects on clinical features and should not be ignored in case histories.
Our results show that the mean incubation period for malaria caused by P. vivax strains from Eurasian temperate zones is statistically and clinically significantly longer than generally considered. This, plus the inherently longer extrinsic incubation period for malaria caused by Old World temperate strains, suggests that an active surveillance period of 31 days after potential exposure is the minimum necessary to capture the 95th percentile of new cases. However, this surveillance interval is balanced by a shorter median time to relapse for Old World temperate strains relative to all New World strains.
These 3 sets of independent measures (i.e., incubation period, time to first relapse, and distribution of the total patient relapses) across the entire course of illness suggest that P. vivax should not be considered a single parasite but is, in fact, several discrete and clinically distinct populations with unique and measurable characteristics. These data, plus previous entomologic and molecular evidence, support the delineation of subspecies within the range of the parasite: P. vivax vivax in the Eastern Hemisphere and P. vivax collins in the Western Hemisphere. This conclusion is supported by a recently published phylogenetic analysis of global P. vivax strains, which shows high diversity and clustering of isolates by hemisphere (34).
The origin of the infecting parasite affects the prophylaxis and treatment of malaria. Infections caused by strains from Korean Peninsula respond to standard doses of primaquine, but even higher doses did not fully suppress strains from New Guinea (Chesson), and infections caused by the Chesson strain required twice the dose of quinine relative to infections by a North American strain (McCoy) (16). Another related study also found large regional differences: infections with Thai strains were more likely to relapse and required higher primaquine dosing relative to infections from India or Brazil (13). The effect of malaria parasite population differences should be considered in the planning and analysis of interventional trials and in potential vaccine trials.
A recent analysis of malaria imported into the United States and Israel found that a large proportion of the case-patients exhibited long periods of latency: of 721 P. vivax case-patients with insufficient/nonexistent antimalarial drug prophylaxis, 46.5% (95% CI 42.8%–50.2%) had an incubation period >2 months, compared with 80.0% (95% CI 77.0%–82.8%) of case-patients with sufficient prophylaxis [authors’ calculations, from (35)]. However, no information was provided about the geographic source of these parasites, and the date of exposure is assumed to be the end of the travel period, making exact calculation of the incubation period impossible. The results from our study are broadly consistent with these values: 31.3% (95% CI 26.2%–36.6%) of case-patients in the time-to-relapse study had an incubation period >8 weeks. The comparability of these antimalarial drug–free populations suggests long-term stability in the global malaria parasite populations and also supports the relevance of these historical challenge studies for modern surveillance programs. The potential for prolonged incubation due to prophylaxis suggests that the estimates from our analysis should be considered as minimum values for travelers returning from vivax-endemic areas who had sufficient malaria prophylaxis.
Control and elimination programs for P. vivax should be reconsidered in light of these findings. Two major stumbling blocks identified during the First Global Malaria Eradication Campaign (1955–1972) were 1) the assumption that control methods could be universally applied and 2) burnout among program staff and funding agencies, resulting from continued surveillance at increasingly lower levels of infection (36). Addressing these issues in the current malaria elimination campaign will require detailed elucidation of the differences in pharmacodynamics among current parasite subpopulations and locale-specific malaria epidemiology, including estimation of incubation periods and the time to relapse to maximize surveillance efficiency.
Our results suggest that considerable complexity among P. vivax populations has been obscured by data aggregation; however, these divisions appear along defined geographic gradients. The long time interval of these studies (1920s–1980s) implies relatively stable parasite populations; however, the effect of greatly increased airplane travel, migration, and population-level antimalarial drug pressure should be explored.
The existence of subpopulations of P. vivax parasites along the division between the Eastern and Western Hemispheres enables conflicting historical and epidemiologic data to be formulated into a consistent and coherent picture, especially with the incorporation of phylogenetic approaches. In addition, parasite origin should be considered in drug prophylaxis and treatment, and the epidemiologic differences in disease caused by P. vivax subpopulations should be more fully elucidated.
Local malaria problems must be solved largely on the basis of local data. It is rarely safe to assume that the variables in one area will behave in the same way as they do in another area, however closely the two regions may seem to resemble each other in topography and climate. Large sums of money have been wasted in attempted malaria control when malariologists have forgotten this fundamental fact. (Paul F. Russell, 1946 [37]).
Acknowledgments
We thank Alex Cook, Ian Mendenhall, Leontine Alkema, Li Yang Hsu, and Michelle Balm for suggestions and critical reviews. We are also indebted to Patrick Royston and Paul Lambert for assistance with the Royston-Parmar models.
Mr Lover, after training as a chemist at Earlham College, Richmond, Indiana, USA, and the University of California, Santa Barbara, California, USA, is now a PhD student at the Saw Swee Hock School of Public Health, National University of Singapore. His main research interests are malariology, infectious disease surveillance, spatial epidemiology, and vectorborne disease.
Prof Coker trained at London University and Columbia University. He heads a research group for the London School of Hygiene and Tropical Medicine in Bangkok and the Infectious Diseases Program at the Saw Swee Hock School of Public Health, National University of Singapore.
References
- Danis K, Baka A, Lenglet A, Van Bortel W, Terzaki I, Tseroni M, Autochthonous Plasmodium vivax malaria in Greece, 2011. Euro Surveill. 2011;16:19993 .PubMedGoogle Scholar
- Armengaud A, Legros F, Quatresous I, Barre H, Valayer P, Fanton Y, A case of autochthonous Plasmodium vivax malaria, Corsica, August 2006. Euro Surveill. 2006;11:E061116.3 .PubMedGoogle Scholar
- Brachman PS. Reemergence of Plasmodium vivax malaria in the Republic of Korea. Emerg Infect Dis. 1998;4:707. DOIPubMedGoogle Scholar
- Lu F, Gao Q, Chotivanich K, Xia H, Cao J, Udomsangpetch R, In vitro anti-malarial drug susceptibility of temperate Plasmodium vivax from Central China. Am J Trop Med Hyg. 2011;85:197–201. DOIPubMedGoogle Scholar
- Hanna JN, Ritchie SA, Brookes DL, Montgomery BL, Eisen DP, Cooper RD. An outbreak of Plasmodium vivax malaria in Far North Queensland, 2002. Med J Aust. 2004;180:24–8 .PubMedGoogle Scholar
- Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77(Suppl):79–87 .PubMedGoogle Scholar
- Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–66. DOIPubMedGoogle Scholar
- White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. DOIPubMedGoogle Scholar
- Myatt AV, Coatney GR. Present concepts and treatment of Plasmodium vivax malaria. AMA Arch Intern Med. 1954;93:191–6. DOIPubMedGoogle Scholar
- Wernsdorfer WH, Gregor IM, editors. Malaria: principles and practice of malariology. New York: Churchill Livingstone; 1988.
- Moon KT, Kim YK, Ko DH, Park I, Shin DC, Kim C. Recurrence rate of vivax malaria in the Republic of Korea. Trans R Soc Trop Med Hyg. 2009;103:1245–9. DOIPubMedGoogle Scholar
- Nishiura H, Lee HW, Cho SH, Lee WG, In TS, Moon SU, Estimates of short- and long-term incubation periods of Plasmodium vivax malaria in the Republic of Korea. Trans R Soc Trop Med Hyg. 2007;101:338–43. DOIPubMedGoogle Scholar
- Goller JL, Jolley D, Ringwald P, Biggs B-A. Regional differences in the response of Plasmodium vivax malaria to primaquine as anti-relapse therapy. Am J Trop Med Hyg. 2007;76:203–7 .PubMedGoogle Scholar
- Winckel CW. Long latency in Plasmodium vivax infections in a temperate zone. Doc Med Geogr Trop. 1955;7:292–8 .PubMedGoogle Scholar
- Krotoski WA. The hypnozoite and malarial relapse. Prog Clin Parasitol. 1989;1:1–19 .PubMedGoogle Scholar
- World Health Organization. Parasitology of malaria: report of a WHO scientific group. Geneva: The Organization; 1969. Report no. 433, WHO technical report series.
- Li J, Collins WE, Wirtz RA, Rathore D, Lal A, McCutchan TF. Geographic subdivision of the range of the malaria parasite Plasmodium vivax. Emerg Infect Dis. 2001;7:35–42. DOIPubMedGoogle Scholar
- Prajapati SK, Joshi H, Shalini S, Patarroyo MA, Suwanarusk R, Kumar A, Plasmodium vivax lineages: geographical distribution, tandem repeat polymorphism, and phylogenetic relationship. Malar J. 2011;10:374. DOIPubMedGoogle Scholar
- Shute PG, Lupascu G, Branzei P, Maryon M, Constantinescu P, Bruce-Chwatt LJ, A strain of Plasmodium vivax characterized by prolonged incubation: the effect of numbers of sporozoites on the length of the prepatent period. Trans R Soc Trop Med Hyg. 1976;70:474–81. DOIPubMedGoogle Scholar
- Song JY, Park CW, Jo YM, Kim JY, Kim JH, Yoon HJ, Two cases of Plasmodium vivax malaria with the clinical picture resembling toxic shock. Am J Trop Med Hyg. 2007;77:609–11 .PubMedGoogle Scholar
- Royston P, Parmar MKB. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–97 . DOIPubMedGoogle Scholar
- Young MD, Ellis JM, Stubbs TH. Some characteristics of foreign vivax malaria induced in neurosyphilis patients. Am J Trop Med Hyg. 1947;27:585–96.
- Coatney GR, Collins WE, Contacos PG. The primate malarias. Washington (DC): United States Government Printing Office; 1971.
- Chernin E. The malariatherapy of neurosyphilis. J Parasitol. 1984;70:611–7 . DOIPubMedGoogle Scholar
- Boyd MF, Kitchen SF. A consideration of the duration of the intrinsic incubation period in vivax malaria in relation to certain factors affecting the parasites. Am J Trop Med Hyg. 1937;1:437–44.
- Baird JK, Schwartz E, Hoffman SL. Prevention and treatment of vivax malaria. Curr Infect Dis Rep. 2007;9:39–46. DOIPubMedGoogle Scholar
- Warrell DA, Gilles HM, editors. Essential malariology, 4th ed. London: Hodder Arnold Publishers; 2002.
- Russell PF. Practical malariology, 2nd ed. London: Oxford University Press; 1963.
- Glynn JR, Bradley DJ. Inoculum size, incubation period and severity of malaria. Analysis of data from malaria therapy records. Parasitology. 1995;110:7–19. DOIPubMedGoogle Scholar
- Winckel CWF. Are the experimental data of therapeutic malaria applicable to conditions obtaining in nature? Am J Trop Med Hyg. 1941;1:789–94.
- Douglas NM, Anstey NM, Buffet PA, Poespoprodjo JR, Yeo TW, White NJ, The anaemia of Plasmodium vivax malaria. Malar J. 2012;11:135. DOIPubMedGoogle Scholar
- Mason J. Patterns of Plasmodium vivax recurrence in a high-incidence coastal area of El Salvador, C.A. Am J Trop Med Hyg. 1975;24:581–5 .PubMedGoogle Scholar
- Contacos PG, Collins WE, Jeffery GM, Krotoski WA, Howard WA. Studies on the characterization of Plasmodium vivax strains from Central America. Am J Trop Med Hyg. 1972;21:707–12 .PubMedGoogle Scholar
- Neafsey DE, Galinsky K, Jiang RHY, Young L, Sykes SM, Saif S, The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat Genet. 2012;44:1046–50. DOIPubMedGoogle Scholar
- Schwartz E, Parise M, Kozarsky P, Cetron M. Delayed onset of malaria—implications for chemoprophylaxis in travelers. N Engl J Med. 2003;349:1510–6. DOIPubMedGoogle Scholar
- Nájera JA. Malaria control: achievements, problems and strategies. Geneva: World Health Organization; 1999. Report no. WHO/CDS/RBM/99.10; WHO/MAL/99.1087.
- Russell PF, West LS, Manwell RD. Practical malariology. Philadelphia: W.B. Saunders; 1946.
Figures
Tables
Cite This ArticleTable of Contents – Volume 19, Number 7—July 2013
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Andrew Lover, Saw Swee Hock School of Public Health, The National University of Singapore, MD3, 16 Medical Dr, Singapore 117597, Singapore; or
Top