Volume 20, Number 11—November 2014
Research
Respiratory Viruses and Bacteria among Pilgrims during the 2013 Hajj
Abstract
Pilgrims returning from the Hajj might contribute to international spreading of respiratory pathogens. Nasal and throat swab specimens were obtained from 129 pilgrims in 2013 before they departed from France and before they left Saudi Arabia, and tested by PCR for respiratory viruses and bacteria. Overall, 21.5% and 38.8% of pre-Hajj and post-Hajj specimens, respectively, were positive for ≥1 virus (p = 0.003). One third (29.8%) of the participants acquired ≥1 virus, particularly rhinovirus (14.0%), coronavirus E229 (12.4%), and influenza A(H3N2) virus (6.2%) while in Saudi Arabia. None of the participants were positive for the Middle East respiratory syndrome coronavirus. In addition, 50.0% and 62.0% of pre-Hajj and post-Hajj specimens, respectively, were positive for Streptococcus pneumoniae (p = 0.053). One third (36.3%) of the participants had acquired S. pneumoniae during their stay. Our results confirm high acquisition rates of rhinovirus and S. pneumoniae in pilgrims and highlight the acquisition of coronavirus E229.
More than 2 million Muslims gather annually in Saudi Arabia for a pilgrimage to the holy places of Islam known as the Hajj. The Hajj presents major public health and infection control challenges. Inevitable overcrowding within a confined area with persons from >180 countries in close contact with others, particularly during the circumambulation of the Kaaba (Tawaf) inside the Grand Mosque in Mecca, leads to a high risk pilgrims to acquire and spread infectious diseases during their time in Saudi Arabia (1), particularly respiratory diseases (2). Respiratory diseases are a major cause of consultation in primary health care facilities in Mina, Saudi Arabia, during the Hajj (3). Pneumonia is a leading cause of hospitalization in intensive care units (4).
Numerous studies have shown a high prevalence of respiratory symptoms among pilgrims (5–7). Respiratory viruses, especially influenza virus, are the most common cause of acute respiratory infections among pilgrims (8–11). We recently reported the acquisition of rhinovirus (5) and Streptococcus pneumoniae infections (12) by French pilgrims during the 2012 Hajj season and highlighted the potential for spread of these infections to home countries of pilgrims upon their return. However, none of the French pilgrims were positive for Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 (13) and 2013 (14).
In this study, we collected paired nasal and throat swab specimens from adult pilgrims departing from Marseille, France to Mecca, Saudi Arabia, for the 2013 Hajj season. The primary objective was to determine the prevalence of the most common respiratory viruses and bacteria upon return of pilgrims from the Hajj. The secondary objective was to evaluate the potential yearly variation of the acquisition of these respiratory pathogens by comparing results from the 2012 and 2013 Hajj seasons.
Participants
Pilgrims who planned to participate in the 2013 Hajj were recruited on September 15, 2013, at a private specialized travel agency in Marseille, France, which organizes travel to Mecca. Potential participants were asked to participate in the study on a voluntary basis if they were ≥18 years of age and were able to provide consent.
Study Design
In this prospective cohort study, participants were sampled and followed up before departing from France (on October 2, 2013) and immediately before leaving Saudi Arabia (on October 24, 2013). Upon inclusion in the study, participants were interviewed by Arabic-speaking investigators who used a standardized pre-travel questionnaire that collected information on the demographic characteristics and medical history of each participant. A post-travel questionnaire that collected clinical data and information on vaccination status and compliance with preventive measures was completed during a face-to-face interview 2 days before the pilgrims returned to France by a single investigator who joined the pilgrims after the Hajj. Health problems that occurred during the pilgrims’ stay were also recorded by a physician who traveled with them during the entire stay in Saudi Arabia, including during the rituals.
Subjective fever was defined as a feverish feeling according to the pilgrims’ report. Influenza-like illness (ILI) was defined as the presence of cough, sore throat, and subjective fever (15). The study protocol was approved by the Aix Marseille Université institutional review board (July 23, 2013; reference no. 2013-A00961–44) and by the Saudi Ministry of Health Ethical Review Committee. The study was performed in accordance with the good clinical practices recommended by the Declaration of Helsinki and its amendments. All participants gave written informed consent.
Respiratory Specimens
Paired nasal and throat swab specimens were collected from each participant by using rigid cotton-tipped swab applicators (Medical Wire and Equipment, Corsham, UK) 10 days (September 22, 2013) before participants departed from France (pre-Hajj specimens) and only 1 day (October 23, 2013) before they left Saudi Arabia (post-Hajj specimens). Nasal and throat swab specimens collected from participants were placed in viral transport media (Virocult and Transwab, respectively; Sigma, St. Louis, MO, USA) at the time of collection and kept at 20°C before being transported to a laboratory in Marseille for storage at −80°C within 48 h of collection.
Detection of Respiratory Viruses
Nasal swab samples were independently tested as described (5) for influenza virus A/H3N2 (16), influenza B virus (16), influenza C virus (17), and A(H1N1)pdm09 virus (18); human adenovirus (19); human bocavirus (20), human cytomegalovirus (21); human coronaviruses (HCoVs); human enterovirus (22); human metapneumovirus (23); human parainfluenza viruses (HPIVs); human parechovirus (24); human respiratory syncytial virus (25); and human rhinovirus (HRV) (26) by using real-time reverse transcription PCRs. HCoVs and human HPIVs were detected by using an HCoV/HPIV R-Gene Kit (Argene/bioMérieux, Marcy l’Etoile, France) (27). HCoV-positive samples were then genotyped by using the FTD Respiratory Pathogens 21 Kit (Fast Track Diagnostics, Luxembourg, Luxembourg).
Detection of Respiratory Bacteria
Throat swab samples were independently tested as described (12) by using quantitative real-time PCRs for Streptococcus pneumoniae, Neisseria meningitidis, Bordetella pertussis, and Mycoplasma pneumoniae. Sequences of all primers and probes have been reported (28). In the present study, reactions were performed by using a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
Statistical Analysis
The Pearson χ2 and Fisher exact tests, as appropriate, were used to analyze categorical variables. Statistical analyses were performed by using SPSS software package version 17 (SPSS Inc., Chicago, IL, USA). p values ≤0.05 were considered significant.
Characteristics of Study Participants
A total of 129 persons were invited to participate in the study. All persons agreed to participate in the study and responded to the pre-travel questionnaire. The participants were 77 women (59.7%) and 52 men (40.3%) who had a mean (SD) age of 61.7 (9.8) years (age range 34–85 years) (Table 1). Although most (94.6%) participants were born in northern Africa, most (94.5%) had lived for years in Marseille or the surrounding cities. More than half of the participants (52.7%) reported having ≥1 chronic disease, as described (14).
Clinical Features
All post-travel questionnaires were completed. During the 3-week stay in Saudi Arabia (October 3–24, 2013), most (90.7%) pilgrims had ≥1 respiratory symptom, including cough (86.8%), sore throat (82.9%), rhinorrhea (72.1%), myalgia (50.4%), fever (49.6%), and dyspnea (21.7%), and 47.3% met the criteria for self-reported ILI (41.3% in 2012 vs. 47.3% in 2013; p = 0.325). Onset of respiratory symptoms peaked in the second week (week 41) after the arrival of the pilgrims in Mecca and decreased thereafter. However, 90 (69.8%) pilgrims still had respiratory symptoms before leaving Saudi Arabia at the time of sampling (week 43). Only 1 pilgrim (0.8%) was hospitalized during the stay in Saudi Arabia (for undocumented pneumonia). No deaths occurred.
Regarding preventive measures, 51.2% of participants reported receiving pneumococcal vaccination (Pneumo 23) in the past 5 years, which was significantly higher than the rate in 2012 (35.9% in 2012 vs. 51.2% in 2013; p = 0.013). None had received the 2013 influenza vaccine before departing for the Hajj, but 44.2% reported having received the seasonal influenza vaccine in 2012 (31.8% among participants <65 years of age vs. 65.8% among participants >65 years of age; p = 0.001). During the stay in Saudi Arabia, 53.5% of pilgrims reported either frequent use (9.3%) or occasional use (44.2%) of facemasks; 93.0% used disposable handkerchiefs; 49.6% reported frequent handwashing; and 67.4% used hand sanitizer. ILI symptoms were less frequently reported by persons who reported receiving the influenza vaccine in 2012 compared with reports by unvaccinated persons (34.1% vs. 61.5%, respectively; p = 0.009) (odds ratio 0.32, 95% CI 0.14–0.76). In contrast, none of the other preventive measures was found to be effective in preventing ILI symptoms during the stay in Saudi Arabia.
Detection of Respiratory Viruses
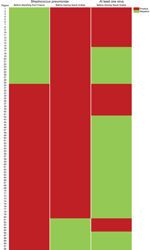
Pre-Hajj and post-Hajj nasal swab specimens were obtained from 121 (93.8%) and 129 (100%) participants, respectively. A total of 26 (21.5%) of 121 pre-Hajj specimens tested were positive for ≥1 virus compared with 50 (38.8%) of 129 post-Hajj specimens tested (p = 0.003) (Table 2). Moreover, 36 (29.8%) participants had acquired ≥1 virus during the stay in Saudi Arabia (Figure 1). The prevalence of human coronavirus E229 (HCoV-E229) was significantly higher in post-Hajj specimens than in pre-Hajj specimens (12.4% vs. 0%; p<0.001). A high prevalence of HRV was observed in pre-Hajj and post-Hajj specimens (14.0% and 14.7%, respectively; p = 0.88). Of 19 participants whose post-Hajj specimens were positive for HRV, 17 (89.5%) had acquired the infection during their stay in Saudi Arabia (Figure 1).
The prevalence of influenza A and B viruses was significantly higher in post-Hajj specimens than in pre-Hajj specimens (7.8% vs. 0%; p = 0.002); further details are described elsewhere (14). Coronaviruses HKU1, NL63, and OC43; human enterovirus; human metapneumovirus; HPIV; and human respiratory syncytial virus were also acquired during the stay in Saudi Arabia by a low proportion of participants (Table 2). Of 50 participants whose post-Hajj specimens were positive for ≥1 respiratory virus, 43 (86.0%) reported ≥1 respiratory symptom during their stay in Saudi Arabia, of whom 37 (86.0%) still had respiratory symptoms at the time of sampling. Also, of 79 participants whose post-Hajj specimens were negative for respiratory viruses, 74 (93.7%) reported ≥1 respiratory symptom during their stay Saudi Arabia, of whom 53 (71.6%) still had respiratory symptoms at the time of sampling. None of the preventive measures was found to be effective in preventing respiratory viruses in post-Hajj specimens.
Detection of Respiratory Bacteria
Pre-Hajj and post-Hajj throat swab specimens were obtained from 126 (97.7%) and 129 (100%) participants, respectively. None of the participants were positive for N. meningitidis, B. pertussis, or M. pneumoniae at any point in the study period (Table 2).
A total of 63 (50.0%) of 126 pre-Hajj specimens tested and 80 (62.0%) of 129 post-Hajj specimens tested were positive for S. pneumoniae (p = 0.053) (Table 2; Figure 2). Of 80 participants whose post-Hajj specimens were positive for S. pneumoniae, 29 (36.3%) had acquired the infection during their stay in Saudi Arabia (Figure 2). In addition, of 63 participants whose pre-Hajj specimens were positive for S. pneumoniae, 12 (19.0%) subsequently had post-Hajj specimens that were negative for S. pneumoniae (Figure 2), of whom 10 (83.3%) reported having received antimicrobial drugs during their stay in Saudi Arabia: 7 received amoxicillin, 2 received amoxicillin and ciprofloxacin, and 1 received azithromycin. Of 80 participants whose post-Hajj specimens were positive for S. pneumoniae, 73 (91.2%) reported ≥1 respiratory symptom during their stay in Saudi Arabia, of whom 56 (76.7%) still had respiratory symptoms at the time of sampling.
Among 66 participants who reported having received a pneumococcal vaccination in the 5 years before traveling to Saudi Arabia, 37 (56.1%) had post-Hajj specimens that were positive for S. pneumoniae. The prevalence of S. pneumoniae in post-Hajj specimens was significantly lower in persons who reported using hand sanitizer during their stay in Saudi Arabia than in remaining participants (55.2% vs. 76.2%; p = 0.021) (odds ratio 0.39, 95% CI 0.17–0.88) and slightly lower in persons who reported more frequent handwashing than usual during their stay in Saudi Arabia than in persons who reported usual handwashing (54.7% vs. 69.2%; p = 0.08).
Of 80 participants whose post-Hajj specimens were positive for S. pneumoniae, 27 (33.8%) were co-infected with ≥1 virus (Figure 2). Of 49 participants whose post-Hajj specimens were negative for S. pneumoniae, 23 (46.9%) were infected with ≥1 virus (33.8% vs. 46.9%; p = 0.14) (Figure 2).
For the second consecutive year, we conducted a prospective longitudinal study of respiratory viruses and bacteria in respiratory specimens collected from a single cohort of pilgrims before departing from Marseille, France, to Mecca, Saudi Arabia, for the Hajj and immediately before leaving Saudi Arabia. By collecting samples from pilgrims before their departure from Saudi Arabia, we were able to rule out acquisition of infections acquired as a result of travel through the international airports of Jeddah, Saudi Arabia, and Istanbul, Turkey, as part of the return trip to Marseille. Close monitoring for respiratory symptoms and compliance with preventive measures was also performed by the investigators accompanying the group.
In this study, we confirmed that performing the Hajj pilgrimage is associated with an increased occurrence of respiratory symptoms in most pilgrims; 8 of 10 pilgrims showed nasal or throat acquisition of respiratory pathogens. This acquisition may have resulted from human-to-human transmission through close contact within the group of French pilgrims because many of them were already infected with HRV or S. pneumoniae before departing from France. Alternatively, the French pilgrims may have acquired these respiratory pathogens from other pilgrims, given the extremely high crowding density to which persons from many parts of the world are exposed when performing Hajj rituals. Finally, contamination originating from an environmental source might have played a role. Sequencing of these pathogens would be required to determine how often new infections were acquired during the stay in Saudi Arabia. However, detection of nasal carriage of coronaviruses other than MERS-CoV and influenza A and B viruses in only the post-Hajj specimens supports the hypotheses that infection occurred during the Hajj.
We confirmed the predominance of HRV and S. pneumoniae among pathogens acquired during the pilgrims’ stay (5,12). We also highlighted acquisition of coronaviruses other than MERS-CoV, most notably HCoV-E229, by pilgrims during the 2013 Hajj pilgrimage. In 2012 and 2013, results of screening for MERS-CoV infection in different cohorts of pilgrims, including the present cohort, were negative (13,14,29). Finally, we found that compared with acquisition of HRV and HCoV-E229, influenza viruses were acquired at a lower frequency among pilgrims.
The present study is a continuation of our previous study in 2012 (5). We extended the investigation to additional viruses, including human bocavirus, human cytomegalovirus, coronaviruses, human parechoviruses, and HPIV, and showed a high frequency of HCoV-E229 infection in pilgrims returning from the Hajj. The prevalence of HRV was lower in 2012 than in 2013, both before departing from France (3.0% in 2012 vs. 14.0% in 2013; p = 0.001) and before leaving Saudi Arabia (8.4% in 2012 vs. 14.7% in 2013; p = 0.092). However, samples that were obtained from pilgrims before departing from France during the 2012 study were stored at room temperature (20°C) for ≤30 days before being processed. This protocol may have resulted in degradation of genetic material, which probably contributed to underestimation of frequencies of infection in 2012. In 2013, all samples collected during the study period were stored at −80°C within 48 h of collection.
The prevalence of S. pneumoniae was also significantly lower in 2012 than in 2013 before pilgrims departed from France (7.3% vs. 50.0%; p<0.001) and before they left Saudi Arabia (19.5% vs. 62.0%; p<0.001). However, in the 2012 study, nasal swab specimens were collected from participants instead of throat swab specimens, which were used in the 2013 study. In addition, the period of the storage of samples before freezing differed between the 2012 and the 2013 studies, as mentioned earlier in this report.
Our results confirm that various respiratory viruses might be acquired by pilgrims during their stay in Saudi Arabia and introduced into home countries of pilgrims on their return, thus contributing to potential international spread of these viruses. However, detection of other human coronaviruses does not enable any conclusions regarding MERS-CoV, for which the available data to date, although limited, indicate different epidemiologic characteristics. We could not demonstrate whether pathogens detected in respiratory specimens were responsible for observed symptoms because nasal carriage was observed in asymptomatic pilgrims in certain instances, and symptoms might have resulted from infection by pathogens that were not investigated in our study. In future studies, checking pilgrims at more frequent intervals might provide useful information. Nevertheless, we believe that Hajj cough likely results from infection of the respiratory tract by various respiratory viruses, including HRV and HCoV-E229, which are known to cause mild or serious lower respiratory tract infections (30,31). However, our results cannot be extrapolated to all pilgrims. A large-scale study based on a similar design and conducted in a large number of pilgrims from many countries would be useful.
We found that pilgrims who had received influenza vaccine in 2012 were less likely to report ILI symptoms during their stay in Saudi Arabia in 2013. Thus, availability of seasonal influenza vaccine for all persons attending the Hajj is crucial. Vaccination with a conjugate pneumococcal vaccine should be considered for persons with medical risk factors for invasive pneumococcal disease. In addition, use of hand sanitizer during the stay in Saudi Arabia was reported by more than two thirds of pilgrims in our survey and was associated with a lower prevalence of S. pneumoniae carriage. Interventional studies are urgently needed that evaluate efficacy of influenza and pneumococcal vaccines and use of hand sanitizer and closely monitor respiratory symptoms and carriage of respiratory pathogens in large cohorts of pilgrims. It is expected that results of such studies will lead to implementation of evidence-based recommendations about preventive measures during the Hajj.
Dr Benkouiten is a researcher at the Institut Hospitalo–Universitaire Méditerranée Infection, Marseille, France. His research interests focus on the epidemiology of respiratory infections in the context of mass gatherings.
Acknowledgment
This study was supported by The Marseille Public Hospitals Authority.
References
- Ahmed QA, Arabi YM, Memish ZA. Health risks at the Hajj. Lancet. 2006;367:1008–15. DOIPubMedGoogle Scholar
- Alzeer AH. Respiratory tract infection during Hajj. Ann Thorac Med. 2009;4:50–3. DOIPubMedGoogle Scholar
- Alzahrani AG, Choudhry AJ, Al Mazroa MA, Turkistani AH, Nouman GS, Memish ZA. Pattern of diseases among visitors to Mina health centers during the Hajj season, 1429 H (2008 G). J Infect Public Health. 2012;5:22–34. DOIPubMedGoogle Scholar
- Mandourah Y, Al-Radi A, Ocheltree AH, Ocheltree SR, Fowler RA. Clinical and temporal patterns of severe pneumonia causing critical illness during Hajj. BMC Infect Dis. 2012;12:117. DOIPubMedGoogle Scholar
- Benkouiten S, Charrel R, Belhouchat K, Drali T, Salez N, Nougairede A, Circulation of respiratory viruses among pilgrims during the 2012 Hajj pilgrimage. Clin Infect Dis. 2013;57:992–1000. DOIPubMedGoogle Scholar
- Meysamie A, Ardakani HZ, Razavi SM, Doroodi T. Comparison of mortality and morbidity rates among Iranian pilgrims in Hajj 2004 and 2005. Saudi Med J. 2006;27:1049–53 .PubMedGoogle Scholar
- Deris ZZ, Hasan H, Sulaiman SA, Wahab MS, Naing NN, Othman NH. The prevalence of acute respiratory symptoms and role of protective measures among Malaysian hajj pilgrims. J Travel Med. 2010;17:82–8. DOIPubMedGoogle Scholar
- Balkhy HH, Memish ZA, Bafaqeer S, Almuneef MA. Influenza a common viral infection among Hajj pilgrims: time for routine surveillance and vaccination. J Travel Med. 2004;11:82–6. DOIPubMedGoogle Scholar
- Rashid H, Shafi S, Booy R, El Bashir H, Ali K, Zambon M, Influenza and respiratory syncytial virus infections in British Hajj pilgrims. Emerg Health Threats J. 2008;1:e2.
- Moattari A, Emami A, Moghadami M, Honarvar B. Influenza viral infections among the Iranian Hajj pilgrims returning to Shiraz, Fars province, Iran. Influenza Other Respir Viruses. 2012;6:e77–9.
- Memish ZA, Assiri AM, Hussain R, Alomar I, Stephens G. Detection of respiratory viruses among pilgrims in Saudi Arabia during the time of a declared influenza A(H1N1) pandemic. J Travel Med. 2012;19:15–21. DOIPubMedGoogle Scholar
- Benkouiten S, Gautret P, Belhouchat K, Drali T, Salez N, Memish ZA, Acquisition of Streptococcus pneumoniae carriage in pilgrims during the 2012 Hajj pilgrimage. Clin Infect Dis. 2014;58:e106–9. DOIPubMedGoogle Scholar
- Gautret P, Charrel R, Belhouchat K, Drali T, Benkouiten S, Nougairede A, Lack of nasal carriage of novel corona virus (HCoV-EMC) in French Hajj pilgrims returning from the Hajj 2012, despite a high rate of respiratory symptoms. Clin Microbiol Infect. 2013;19:E315–7. DOIPubMedGoogle Scholar
- Gautret P, Charrel R, Benkouiten S, Belhouchat K, Nougairede A, Drali T, Lack of MERS coronavirus but prevalence of influenza virus in French pilgrims after 2013 Hajj. Emerg Infect Dis. 2014;20:728–30. DOIPubMedGoogle Scholar
- Rashid H, Shafi S, El Bashir H, Haworth E, Memish ZA, Ali KA, Influenza and the Hajj: defining influenza-like illness clinically. Int J Infect Dis. 2008;12:102–3. DOIPubMedGoogle Scholar
- van Elden LJ, Nijhuis M, Schipper P, Schuurman R, van Loon AM. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. DOIPubMedGoogle Scholar
- Faux C. Influenza type C. PCR methodology. In: Schuller M, Sloots TP, James GS, Halliday CL, Carter IW, editors. PCR for clinical microbiology: an Australian and international perspective. London: Springer; 2010. p. 311–2.
- Duchamp MB, Casalegno JS, Gillet Y, Frobert E, Bernard E, Escuret V, Pandemic A(H1N1)2009 influenza virus detection by real time RT-PCR: is viral quantification useful? Clin Microbiol Infect. 2010;16:317–21. DOIPubMedGoogle Scholar
- Heim A, Ebnet C, Harste G, Pring-Akerblom P. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J Med Virol. 2003;70:228–39. DOIPubMedGoogle Scholar
- Lu X, Chittaganpitch M, Olsen SJ, Mackay IM, Sloots TP, Fry AM, Real-time PCR assays for detection of bocavirus in human specimens. J Clin Microbiol. 2006;44:3231–5. DOIPubMedGoogle Scholar
- Griscelli F, Barrois M, Chauvin S, Lastere S, Bellet D, Bourhis JH. Quantification of human cytomegalovirus DNA in bone marrow transplant recipients by real-time PCR. J Clin Microbiol. 2001;39:4362–9. DOIPubMedGoogle Scholar
- Tan CY, Ninove L, Gaudart J, Nougairede A, Zandotti C, Thirion-Perrier L, A retrospective overview of enterovirus infection diagnosis and molecular epidemiology in the public hospitals of Marseille, France (1985–2005). PLoS ONE. 2011;6:e18022. DOIPubMedGoogle Scholar
- Maertzdorf J, Wang CK, Brown JB, Quinto JD, Chu M, de Graaf M, Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–6. DOIPubMedGoogle Scholar
- Bennett S, Harvala H, Witteveldt J, McWilliam Leitch EC, McLeish N, Templeton K, Rapid simultaneous detection of enterovirus and parechovirus RNAs in clinical samples by one-step real-time reverse transcription-PCR assay. J Clin Microbiol. 2011;49:2620–4 and. DOIPubMedGoogle Scholar
- van Elden LJ, van Loon AM, van der Beek A, Hendriksen KA, Hoepelman AI, van Kraaij MG, Applicability of a real-time quantitative PCR assay for diagnosis of respiratory syncytial virus infection in immunocompromised adults. J Clin Microbiol. 2003;41:4378–81. DOIPubMedGoogle Scholar
- Lu X, Holloway B, Dare RK, Kuypers J, Yagi S, Williams JV, Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46:533–9. DOIPubMedGoogle Scholar
- Pillet S, Lardeux M, Dina J, Grattard F, Verhoeven P, Le Goff J, Comparative evaluation of six commercialized multiplex PCR kits for the diagnosis of respiratory infections. PLoS ONE. 2013;8:e72174. DOIPubMedGoogle Scholar
- Cohen-Bacrie S, Ninove L, Nougairède A, Charrel R, Richet H, Minodier P, Revolutionizing clinical microbiology laboratory organization in hospitals with in situ point-of-care. PLoS ONE. 2011;6:e22403. DOIPubMedGoogle Scholar
- Memish ZA, Assiri A, Almasri M, Alhakeem RF, Turkestani A, Al Rabeeah AA, Prevalence of MERS-CoV nasal carriage and compliance with the Saudi health recommendations among pilgrims attending the 2013 Hajj. J Infect Dis. 2014. In press and. DOIPubMedGoogle Scholar
- Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. 2013;26:135–62. DOIPubMedGoogle Scholar
- Graham RL, Donaldson EF, Baric RS. A decade after SARS: strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013;11:836–48. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 20, Number 11—November 2014
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Philippe Gautret, Centre Hospitalier Universitaire Nord, Chemin des Bourrely, 13915 Marseille, France
Top