Volume 20, Number 12—December 2014
Dispatch
Putative New West Nile Virus Lineage in Uranotaenia unguiculata Mosquitoes, Austria, 2013
Abstract
West Nile virus (WNV) is becoming more widespread and markedly effecting public health. We sequenced the complete polyprotein gene of a divergent WNV strain newly detected in a pool of Uranotaenia unguiculata mosquitoes in Austria. Phylogenetic analyses suggest that the new strain constitutes a ninth WNV lineage or a sublineage of WNV lineage 4.
West Nile virus (WNV), the most widespread flavivirus, is distributed throughout Africa, Asia, Europe, and Australia, and since 1999, WNV has also been present in the Americas (1). Within the last 2 decades, WNV infection has caused an increasing number of cases of neuroinvasive disease in humans and become a substantial public health problem (1).
Up to 8 lineages of WNV, based on genetic differences, have been proposed (1,2) (Table 1). Lineage 1 is widely distributed and further divided into lineage 1a, which includes the American strains; lineage 1b, which is also referred to as Kunjin virus and mainly described in Australia; and lineage 1c, which is also referred to as lineage 5 and comprises isolates from India. Lineage 2 has been detected in Africa and several parts of Europe, lineage 3 (Rabensburg virus) has been isolated only in the Czech Republic, and lineage 4 has been reported from Russia (3). A putative sixth lineage, based on a small genome fragment, has been described from Spain (4), and putative lineages 7 (Koutango virus) and 8 have been reported from Senegal (2).
WNV is maintained in an enzootic cycle between mosquitoes and wild birds (1). In 2013, ≈100 Uranotaenia unguiculata Edwards, 1913, mosquitoes were trapped during mosquito-monitoring projects at Lake Neusiedl-Seewinkel National Park in Austria and near Sedlec in the Czech Republic. In Russia, Ur. unguiculata mosquitoes have been described as hosting lineage 4 WNV strains (A. Platonov, unpub. data) (GenBank accession nos. FJ154906–49 and FJ159129–31). To determine whether Ur. unguiculata mosquitoes in Austria and the Czech Republic also host WNV, we investigated the mosquitoes collected in 2013 for the presence of WNV, focusing on lineage 4 viruses.
During May–October 2013, ≈11,300 female mosquitoes belonging to 13 species were trapped at 4 sites in Lake Neusiedl-Seewinkel National Park in Burgenland State, Austria. Mosquito species were determined according to morphologic criteria (5). Individual mosquitoes were pooled by species and collection site and date. A total of 47 Ur. unguiculata mosquitoes were collected in Austria (12 pools, 1–12 mosquitoes/pool). The relative abundance of Ur. unguiculata mosquitoes among the total collected in Austria was 0.42%. During August 2013, ≈39,000 mosquitoes were trapped at 2 fish ponds (Nesyt and Novy) in Sedlec, Czech Republic, near the border with northeastern Austria. A total of 47 female Ur. unguiculata mosquitoes were grouped into 4 pools (2 with 1 mosquito each, 1 with 4 mosquitoes, and 1 with 41 mosquitoes). The relative abundance of Ur. unguiculata mosquitoes among the total collected in the Czech Republic was 0.12%.
The mosquito pools were homogenized in RNase-free water, and RNA was extracted by using the QIAamp Viral RNA Mini Kit (QIAGEN, Valencia, CA, USA). The samples were screened for the presence of flavivirus nucleic acid by reverse transcription PCR, using universal flavivirus primers MAMD (6) and CFD2 (6,7) for amplification of a partial nonstructural protein (NS) 5 sequence. Results were negative for the samples from Czech Republic. One pooled sample from Austria was positive; the pool contained 9 mosquitoes that had been captured in late August in Illmitz, a village east of Lake Neusiedl (47.769997°N, 16.752887°E). We obtained the complete polyprotein coding sequence and partial 5′ and 3′ noncoding ends of this novel WNV strain (GenBank accession no. KJ831223), which was designated West Nile virus-Uranotaenia unguiculata-Lake Neusiedl-Austria-2013 (WNV-Uu-LN-AT-2013). Primer sequences and amplification protocols are available upon request.
The complete polyprotein gene sequence of WNV-Uu-LN-AT-2013 shares a maximum identity of ≈83% with lineage 4 WNV strains isolated from Ur. unguiculata mosquitoes and Dermacentor marginatus ticks in Russia (3). At the amino acid level, the entire polyproteins of WNV-Uu-LN-AT-2013 and the lineage 4 strains from Russia share ≈96% identity (Table 2). Compared with the Russian lineage 4 strains, a 1,813-nt fragment of the NS5-coding sequence for the putative lineage 6 WNV, isolated from Culex pipiens mosquitoes in Spain (4), shares slightly higher nucleotide and amino acid identities with WNV-Uu-LN-AT-2013 (Table 2).
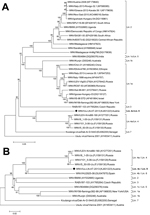
Phylogenetic neighbor-joining trees were generated with MEGA5 software, using ClustalW alignments, 1,000 replicates for bootstrap testing, and evolutionary distances computation with the p-distance model (8). One phylogenetic tree was constructed on the basis of the complete polyprotein-encoding nucleotide sequences of 32 WNV strains representing all previously described lineages for which complete polyprotein-encoding sequences are available. This tree also showed a close relationship between WNV-Uu-LN-AT-2013 and the lineage 4 WNV strains from Russia; however, the newly identified strain forms a distinct branch (Figure, panel A). A second phylogenetic analysis that included the proposed lineage 6 virus from Spain and that was based on 1,813-nt fragments of NS5 showed a close grouping of WNV-Uu-LN-AT-2013 virus from Austria, the virus from Spain, and the lineage 4 viruses from Russia; similarity was slightly higher between the viruses from Austria and Spain (Figure, panel B).
WNV-Uu-LN-AT-2013 encodes a polyprotein of 3,432 aa. The envelope protein carries 1 putative N-linked glycosylation site at asparagine residue N-154, which has been associated with increased WNV pathogenicity and neuroinvasiveness (9). The 3 highly conserved N-linked glycosylation sites at NS1 positions N-130, N-175, and N-207 in WNV strains were also calculated for WNV-Uu-LN-AT-2013 by using NetNGlyc 1.0 software (http://www.cbs.dtu.dk/services/NetNGlyc/). Glycosylation of NS1 at these 3 positions has been implicated in neuroinvasiveness (10), as has proline at NS1 aa position 250 (11), which is also present in WNV-Uu-LN-AT-2013. The NS2A-encoding nucleotide region contains a foo motif, which can mediate production of NS1′, a variant of NS1 that plays a role in neuroinvasiveness (12). A fifo motif, which has been described for the nonpathogenic mosquito-specific flaviviruses (13), could not be determined for WNV-Uu-LN-AT-2013.
WNV lineages 1–4 and putative lineage 6 have been detected in Europe, but only WNV lineage 1a has spread across the American continents. Circulation of such a genetically diverse group of WNV strains in Europe may partly explain the epidemiologic differences observed between WNV disease in Europe and the Americas. In Europe, the presence of less pathogenic WNV strains may inhibit the spread of more pathogenic strains.
We propose that the WNV-Uu-LN-AT-2013 strain from Austria either constitutes a new lineage (lineage 9) or can be grouped into lineage 4 as sublineage 4c, with the strains from Russia and Spain as sublineages 4a and 4b, respectively. However, the short sequence available for the strain from Spain does not allow a clear-cut conclusion to be drawn with regard to lineage 4. We suggest that future designation of new WNV lineages should be restricted to viruses for which at least the complete polyprotein gene sequences have been determined. In addition, rules for defining virus lineages should be established by the International Committee on Taxonomy of Viruses.
Strain WNV-Uu-LN-AT-2013 has been detected only in Ur. unguiculata mosquitoes. These mosquitoes are mainly distributed in the southern half of Europe (5); in eastern Europe, they have spread from southern Ukraine and the Volga Delta through middle and southwestern Asia to Iran and Pakistan (5). In the Lake Neusiedl area of Austria, Ur. unguiculata mosquitoes seem to be an indigenous species, which was first reported in 1970 (14). In the Czech Republic, Ur. unguiculata mosquitoes have been detected only in Moravia, in the southern part of the country (15). Although there are anecdotal reports of Ur. unguiculata mosquitoes feeding on mammals, including humans, they feed mainly on amphibians and reptiles (5).
The pathogenicity of strain WNV-Uu-LN-AT-2013 in humans and animals has not been elucidated. Genetic data show that the strain carries typical WNV pathogenicity markers and suggest that WNV-Uu-LN-AT-2013 is not restricted to mosquitoes. Additional monitoring studies involving cell culture and animal isolation experiments are necessary to evaluate the pathogenic potential of this virus for humans and animals.
Dr Pachler is a postdoctoral researcher at the Institute of Virology, University of Veterinary Medicine, Vienna, Austria. Her research interests include the molecular biology of emerging and vectorborne viruses.
Acknowledgments
We thank Oldřich Šebesta for collecting and identifying mosquitoes in the Czech Republic and Jolanta Kolodziejek for her support.
This study was partially funded by support from the European Commission (grants HEALTH.2010.2.3.3-3 Project 261391 EuroWestNile [http://www.eurowestnile.org] and FP7-261504 EDENext [http://www.edenext.eu], and is catalogued by the EDENext Steering Committee as EDENext228).
References
- Gray TJ, Webb CE. A review of the epidemiological and clinical aspects of West Nile virus. Int J Gen Med. 2014;7:193–203.PubMedGoogle Scholar
- Fall G, Diallo M, Loucoubar C, Faye O, Sall AA. Vector competence of Culex neavei and Culex quinquefasciatus (Diptera: Culicidae) from Senegal for lineages 1, 2, Koutango and a putative new lineage of West Nile virus. Am J Trop Med Hyg. 2014;90:747–54 . DOIPubMedGoogle Scholar
- Lvov DK, Butenko AM, Gromashevsky VL, Kovtunov AI, Prilipov AG, Kinney R, West Nile virus and other zoonotic viruses in Russia: examples of emerging–reemerging situations. Arch Virol Suppl. 2004;18:85–96 .PubMedGoogle Scholar
- Vázquez A, Sánchez-Seco MP, Ruiz S, Molero F, Hernández L, Moreno J, Putative new lineage of West Nile virus, Spain. Emerg Infect Dis. 2010;16:549–52 . DOIPubMedGoogle Scholar
- Becker N, Petrič D, Zgomba M, Boase C, Madon M, Dahl C, Mosquitoes and their control, 2nd ed. Heidelberg (Germany): Springer Verlag; 2010. p. 91–111, 312–4.
- Scaramozzino N, Crance J-M, Jouan A, DeBriel DA, Stoll F, Garin D. Comparison of Flavivirus universal primer pairs and development of a rapid, highly sensitive heminested reverse transcription–PCR assay for detection of flaviviruses targeted to a conserved region of the NS5 gene sequences. J Clin Microbiol. 2001;39:1922–7 . DOIPubMedGoogle Scholar
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73–83 .PubMedGoogle Scholar
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9 . DOIPubMedGoogle Scholar
- Shirato K, Miyoshi H, Goto A, Ako Y, Ueki T, Kariwa H, Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol. 2004;85:3637–45 . DOIPubMedGoogle Scholar
- Whiteman MC, Wicker JA, Kinney RM, Huang CYH, Solomon T, Barrett ADT. Multiple amino acid changes at the first glycosylation motif in NS1 protein of West Nile virus are necessary for complete attenuation for mouse neuroinvasiveness. Vaccine. 2011;29:9702–10 . DOIPubMedGoogle Scholar
- Hall RA, Khromykh AA, Mackenzie JM, Scherret JH, Khromykh TI, Mackenzie JS. Loss of dimerisation of the nonstructural protein NS1 of Kunjin virus delays viral replication and reduces virulence in mice, but still allows secretion of NS1. Virology. 1999;264:66–75 . DOIPubMedGoogle Scholar
- Melian EB, Hinzman E, Nagasaki T, Firth AE, Wills NM, Nouwens AS, NS1′ of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J Virol. 2010;84:1641–7 . DOIPubMedGoogle Scholar
- Firth AE, Blitvich BJ, Wills NM, Miller CL, Atkins JF. Evidence for ribosomal frameshifting and a novel overlapping gene in the genomes of insect-specific flaviviruses. Virology. 2010;399:153–66 . DOIPubMedGoogle Scholar
- Aspöck H, Kunz C, Pretzmann G. Seasonal distribution of mosquitoes and its relation to the appearance of mosquito-borne viruses in the eastern part of the Neusiedlersee area (eastern Austria) [in German]. Zentralbl Bakteriol [Orig]. 1970;214:160–73 .PubMedGoogle Scholar
- Šebesta O, Halouzka J, Hubálek Z, Juřicová Z, Rudolf I, Šikutová S, Mosquito (Diptera: Culicidae) fauna in an area endemic for West Nile virus. J Vector Ecol. 2010;35:156–62 . DOIPubMedGoogle Scholar
Figure
Tables
Cite This Article1Current affiliation: Paracelsus Medical University, Salzburg, Austria.
Table of Contents – Volume 20, Number 12—December 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Norbert Nowotny, Viral Zoonoses, Emerging and Vector-Borne Infections Group, Institute of Virology, University of Veterinary Medicine Vienna, Veterinaerplatz 1, 1210 Vienna, Austria; and
Top