Volume 20, Number 2—February 2014
Research
Human Antibody Responses to Avian Influenza A(H7N9) Virus, 2013
Figure 3
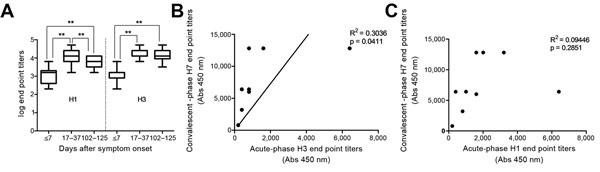
Figure 3. Association between antibody responses against H7 and seasonal subtypes in patients infected with influenza A(H7N9) virus, ChinaA) Levels of IgG against H1 and H3 in serum samples after symptom onsetIgG in samples taken at acute-phase (≤7 days), convalescent-phase (17–37days,) and 102–125 days after symptom onset were titrated by ELISA with recombinant H1 and H3 hemagglutinin antigens, respectivelyIgG titers were transformed to log10Bars indicate SEB and C) Correlation between IgG against H3 (B) and H1 (C) in acute-phase serum and against H7 in convalescent-phase serum**p<0.01.
1These authors contributed equally to this article and are co–first authors.
2These authors contributed equally to this article and are co–senior authors.
Page created: January 17, 2014
Page updated: January 17, 2014
Page reviewed: January 17, 2014
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.