Volume 20, Number 3—March 2014
CME ACTIVITY - Synopsis
Invasive Fungal Infections after Natural Disasters
Search Strategy and Selection Criteria
Pathogenic Fungi and the Environment
Respiratory Fungal Infections after Disasters
Soft Tissue Fungal Infections after Disasters
Health Care–associated Fungal Infections after Disasters
Indoor Mold Exposures after Disasters
Disasters, Fungi, and Global Climate Change
Conclusions
CME Follow Up
Cite This Article
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/eid; (4) view/print certificate.
Release date: February 13, 2014; Expiration date: February 13, 2015
Learning Objectives
Upon completion of this activity, participants will be able to:
• Analyze the risk for fungal infections after natural disasters, including coccidioidomycosis
• Distinguish features of water-related fungal infections after natural disasters
• Assess the prevalence and microbiological characteristics of soft tissue fungal infections after natural disasters
• Evaluate the health risks associated with exposure to mold after natural disasters.
CME Editor
Jean Michaels Jones, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Jean Michaels Jones has disclosed no relevant financial relationships.
CME Author
Charles P. Vega, MD, Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine. Disclosure: Charles P. Vega, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Kaitlin Benedict, MPH, and Benjamin J. Park, MD, have disclosed no relevant financial relationships.
Abstract
The link between natural disasters and subsequent fungal infections in disaster-affected persons has been increasingly recognized. Fungal respiratory conditions associated with disasters include coccidioidomycosis, and fungi are among several organisms that can cause near-drowning pneumonia. Wound contamination with organic matter can lead to post-disaster skin and soft tissue fungal infections, notably mucormycosis. The role of climate change in the environmental growth, distribution, and dispersal mechanisms of pathogenic fungi is not fully understood; however, ongoing climate change could lead to increased disaster-associated fungal infections. Fungal infections are an often-overlooked clinical and public health issue, and increased awareness by health care providers, public health professionals, and community members regarding disaster-associated fungal infections is needed.
The potential for adverse health events after natural disasters is well recognized and comprises various challenges for public health (1). The World Health Organization defines a disaster as a disruption of society resulting in widespread human, material, or environmental loss that exceeds the affected society’s ability to cope by using local resources (2). Natural disasters can be broadly classified into 3 groups: geophysical (e.g., earthquakes, volcanic eruptions, and tsunamis), hydrometeorological (e.g., floods, hurricanes, and tornadoes), and geomorphological (e.g., landslides and avalanches). Specific social, economic, and cultural settings create a unique set of circumstances for every disaster, and the immediate causes of illness and death (such as blunt trauma, lacerations, crush injuries, suffocation, and drowning) vary according to the type of event (1).
Infectious disease outbreaks after natural disasters are uncommon. However, features of the post-impact and recovery phases of disasters, such as population displacement, low vaccine coverage for vaccine-preventable diseases, inadequate sanitation and hygiene infrastructure, and limited access to health care services, can interact to increase the risk for transmission of infectious diseases that were previously established in the affected area (3). Disaster-associated fungal infections are similarly uncommon, but they are becoming increasingly recognized and are typically attributable to the impact phase of a disaster because such infections primarily result from inhalation or cutaneous inoculation of fungal spores directly from the environment (3,4). During a disaster, pathogenic fungi can be displaced from their natural habitats, which could increase their environmental concentration or introduce them to areas where they would not normally be found, resulting in contact with injured persons and potentially causing fungal infections. To increase awareness of these events among health care providers and public health officials, we summarize the known occurrences of fungal infections associated with natural disasters (Table).
The online literature databases PubMed and Google Scholar were searched for English-language articles about fungal infections related to natural disasters that were published as of April 2013. Search terms included combinations of “disaster,” “natural disaster,” “tornado,” “hurricane,” “earthquake,” “tsunami,” and “flood” with “fungal infection,” “fungal disease,” “fungus,” or “mold.” References cited in relevant articles were also reviewed, and the authors’ personal records were searched for conference abstracts.
Of the 1.5 million species of fungi on Earth, ≈300 are known human pathogens (20). Pathogenic fungi exist in a broad range of natural habitats but are believed to be more common in subtropical and tropical areas of the world, probably because of environmental restrictions on their growth or propagation (20). Known geographic habitats of some pathogenic fungi (for example, Blastomyces, Coccidioides, and Histoplasma) are well defined, but others (such as Aspergillus and other molds) are thought to be ubiquitous. The abundance and distribution (and therefore, potential to cause disease) of environmental fungi probably depend on climatic or environmental factors such as ambient temperature and moisture (20). Examples include coccidioidomycosis incidence in Arizona, which has been shown to correlate with hot, dry conditions; blastomycosis clusters observed in association with rainfall after periods of decreased precipitation; and incidence of Penicillum marneffei penicilliosis, an opportunistic infection which is endemic to Southeast Asia and increases in incidence during rainy months (21,22). Aspergillosis and other invasive mold infections have also been postulated to correlate with seasons or weather patterns in some geographic areas (23).
Although seasonal changes and weather effects probably play a role in the growth and distribution of many pathogenic fungi, environmental disruption is a key factor in the dispersal of these organisms and their resulting potential for causing infection. Both small-scale earth-disrupting activities, such as excavation or construction, and events affecting larger areas, such as earthquakes (16), tsunamis (5–7,10–15,24,25), and tornadoes (4), have been linked to the occurrence of fungal infections. Natural disasters can cause large-scale disruption of fungal habitats, which can lead to clusters of respiratory, cutaneous, or other forms of fungal disease.
Studies of clinical specimens collected from persons injured during disasters highlight the diversity of potential fungal pathogens in the natural environment. For example, after an 8.0-magnitude earthquake in Wenchuan, China, during 2008, 19 strains of fungi were identified in wound, sputum, and blood cultures from 123 injured persons (26), and fungi accounted for 7.6% of clinical isolates obtained from 42 patients with crush syndrome (27). Similarly, after a 1970 tornado in Texas, United States, fungi were identified in 8 (6.5%) of 124 wound isolates from 24 hospitalized patients (Fusarium, unspecified yeast, Rhodotorula, Aspergillus, Hormodendrum [now Cladosporium], and Cephalosporium), and in 4 (10.5%) of 38 wound isolates from 23 ambulatory patients (28). In each of these reports, multiple organisms were isolated from many of the patients, and the fungi recovered may not necessarily have been agents of infection.
Airborne
Inhalation is a common route for fungal infections. Fungi are known to cause respiratory infections ranging from asymptomatic to life-threatening, depending on the pathogen and host characteristics. Coccidioides spp. are dimorphic fungi that grow in semiarid soil and are endemic to the southwestern United States, northern Mexico, and parts of South America. Two instances of disaster-associated coccidioidomycosis have been described.
An outbreak of coccidioidomycosis after the January 1994 earthquake in Northridge, California, United States, was 1 of few known examples of any infectious disease outbreak directly related to a geophysical disaster (3). Coccidioides spores were presumably aerosolized as a consequence of the earthquake, its aftershocks, and associated landslides and were dispersed by the resulting widespread dust clouds (16). In Ventura County, California, 203 outbreak-associated coccidioidomycosis cases were identified, and investigators found that dust exposure was substantially associated with acute illness (16). Fungal infection may not have been considered in the initial diagnoses in this outbreak; 93% of case-patients received >1 antibacterial drug before coccidioidomycosis was diagnosed (16).
Another coccidioidomycosis outbreak occurred after a severe dust storm in the southern San Joaquin Valley of California in December 1977 (18). The storm originated near Bakersfield, an area to which coccidioidomycosis is highly endemic, and covered nearly 90,000 km2, an area larger than the state of Maine (18). In Sacramento County, an area to which the disease was not previously considered to be endemic, 115 cases of coccidioidomycosis were attributed to the dust storm, including 16 cases of disseminated disease (18). Eighteen additional cases were identified at a US Navy air station in Kings County (19), and other California counties affected by the storm saw more coccidioidomycosis cases than usual; for example, Kern County recorded 134 cases during January and February 1978, compared with 17 cases during those months in the previous year (29).
Near-drowning
Drowning and near-drowning are common during and after disaster-related flooding (1). Aspiration of contaminated or debris-laden water can lead to sinus and pulmonary infections; aspiration pneumonia is often referred to as “tsunami lung” in post-tsunami settings (16,24). Tsunami lung can be caused by bacteria, fungi, or both. Pseudallescheria boydii (asexual form, Scedosporium apiospermum) is hypothesized to be the most common fungal pathogen associated with near-drowning, although this finding has not been studied specifically in the context of disasters (30). Information about post-disaster Scedosporium lung infection is limited to a small number of case reports; these reports also document the organism’s propensity to progress to central nervous system infection, even in immunocompetent hosts (6,11). Other fungal pathogens, such as Aspergillus, have also been implicated as agents of tsunami lung; after the 2011 Great East Japan Earthquake and subsequent tsunami, a previously healthy near-drowning victim who later died was found to have pneumonia caused by Aspergillus fumigatus and evidence of multiorgan disseminated aspergillosis upon autopsy (5). The report describes delays in specimen transportation and receipt of culture results caused by the aftermath of the earthquake, which led to a delay in diagnosis and treatment (5).
Evidence of tsunami lung also was also apparent after the December 2004 earthquake and tsunami in the Indian Ocean, which killed >200,000 persons (3). In Sri Lanka, acute respiratory issues attributed to post-aspiration pneumonitis and polymicrobial pneumonia that were not related to communicable illnesses were the most frequent medical problems after this disaster (31). In Banda Aceh, Indonesia, several patients with necrotizing pneumonia, who did not respond to broad-spectrum antibacterial drugs, probably had polymicrobial infections that may have included fungal organisms (24). However, limited diagnostic capacity for fungi may have affected the ability to identify the potential role of fungi in these infections. A report from Germany demonstrated that among a cohort of 17 tourists injured during the tsunami, all had clinical and radiologic evidence of aspiration pneumonitis and pneumonia; Candida albicans and A. fumigatus were isolated from the respiratory tract of several patients, although it is unclear whether the isolation of these organisms represented true infection or colonization (15).
The risk for wound infections after a natural disaster is high when wounds are contaminated with water, soil, or debris (32). In addition, damage to the local health care infrastructure can compromise the ability to properly irrigate contaminated wounds with sterile solution or promptly treat injured persons with topical or systemic antimicrobial drugs (32). These factors can result in severe, often polymicrobial, infections of otherwise relatively minor injuries (32). Although most documented disaster-associated soft tissue infections are bacterial (typically gram-negative pathogens such as Aeromonas, Escherichia coli, and Klebsiella) (33), fungal wound infections can also occur, and they could be under-recognized because they can be clinically similar to bacterial infections, particularly during the early stages of infection.
Mucormycosis, caused by fungi that belong to the order Mucorales, is perhaps the most recognized example of post-disaster fungal soft tissue infection. Necrotizing fasciitis can result, and case-fatality rates of ≈30% are frequently described, although early diagnosis and treatment has been shown to lead to better outcomes (34). The first documented instance of disaster-associated mucormycosis occurred after the 1985 volcanic eruption in Armero, Colombia, which caused an estimated 23,000 deaths and ≈4,500 injuries (17). According to a report of 38 patients with necrotizing lesions who were hospitalized after the volcano, 8 patients had infections caused by the mucormycete Rhizopus arrhizus (oryzae) (17).
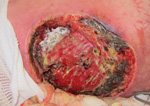
Similarly, a cluster of mucormycosis cases caused by Apophysomyces trapeziformis occurred among 13 persons who were severely injured in the May 22, 2011, tornado in Joplin, Missouri, United States (4). Penetrating trauma and an increased number of wounds were shown to be independent risk factors for mucormycosis. Whole-genome sequencing of A. trapeziformis isolates from case-patients’ wounds (Figure) showed 4 nonidentical but closely related strains of A. trapeziformis. This finding, considered with case-patients’ receipt of medical care at different hospitals, suggested that the infections were acquired from the natural environment as a result of exposure to organic matter and water, which are likely reservoirs for mucormycetes (4).
In addition to the 2 clusters described, several isolated cases of post-disaster soft tissue mucormycosis have been reported, notably among persons injured during the 2004 Indian Ocean tsunami (13–15). These reports illustrate some of the clinical challenges associated with soft tissue mucormycosis, caused by organisms that may initially appear indistinguishable from other types of wound infections but require aggressive treatment with intravenous antifungal medication and surgical debridement (13,14).
Other agents of fungal soft tissue infections in survivors injured during the 2004 Indian Ocean tsunami include Fusarium (which later caused systemic infection) in 1 tourist (15) and Cladophialophora bantiana in 2 other tourists (10). Subcutaneous C. bantiana infection has also been associated with a tornado-associated injury in which the patient was inoculated by a contaminated wood splinter but did not have symptoms until ≈16 years later (35). Soft tissue fungal infections have also been documented among persons who were not directly injured during a disaster but who sustained minor trauma while performing post-disaster tasks: in Texas after Hurricane Ike in 2008, chromoblastomycosis was diagnosed in 3 patients, all of whom had histories of cancer and all of whom described clearing brush and fallen trees near their homes after the storm (8).
Although respiratory and cutaneous infections are the most commonly described forms of fungal infection after natural disasters, other, more invasive fungal infections have also been observed. An outbreak of Aspergillus meningitis after the 2004 Indian Ocean tsunami was associated with the use of spinal anesthesia for cesarean section infant delivery for 6 previously healthy women in Sri Lanka (12). The first 5 case-patients were initially treated for bacterial meningitis, but the discovery of Aspergillus during the post-mortem examination of the index case-patient led to the use of amphotericin B and voriconazole in the surviving case-patients (12). Investigation of various medical supplies revealed that syringes from a central storage facility were contaminated with A. fumigatus, probably as a result of suboptimal storage conditions in a humid warehouse (12).
Disaster-induced water damage to structures can create moist environments that can promote indoor fungal growth, but the extent to which damp indoor spaces and mold growth affect human health remains somewhat ambiguous (36). A 2004 report by the Institute of Medicine found sufficient evidence of association between indoor mold exposure and upper respiratory tract symptoms, cough, and wheezing, and evidence of an association between indoor mold exposure and some noninfectious health conditions that included asthma symptoms in persons with asthma and hypersensitivity pneumonitis in some groups of people (36). Although the report found no association between indoor mold exposure and invasive infection in healthy persons, there was evidence to support a link between exposure to Aspergillus and aspergillosis in severely immunocompromised persons (36).
Few data clearly demonstrate that indoor mold exposures increase the risk for invasive infection in post-disaster settings. Flooding lasted for weeks after Hurricanes Katrina and Rita made landfall on the US Gulf Coast in August and September 2005, respectively, leading to visible mold growth in 46% of 112 inspected homes (37). Despite these high levels of indoor mold growth documented in some areas, 1 study showed no elevated risk for fungal infections among immunocompromised patients exposed to water-damaged buildings after Hurricane Katrina; 1 patient, 1.2% of the profoundly immunocompromised study population, had a mold infection (caused by a Cladosporium sp.), which resolved without antifungal treatment (9). Colonization in the absence of related clinical symptoms was observed in persons who returned to their water-damaged homes after Hurricanes Rita and Katrina: the mucormycete Syncephalastrum was detected in various clinical specimens from 8 persons whose self-reported exposures to mold ranged from none to heavy, but none had evidence of invasive infection (38).
After the 2011 Great East Japan Earthquake and subsequent tsunami, a medical relief team observed unexplained chronic cough among a group of previously healthy persons living in a temporary refuge (25). Fungal cultures of sputum samples from 6 persons yielded Aspergillus fumigatus, A. flavus, and basidiomycetous fungi; culture plates exposed inside the refuge showed a similar fungal profile, suggesting that the indoor environment may have played a role in the patients’ infections (25). Although the authors of that report state that the patients’ coughs resolved after treatment with the antifungal itraconazole, the extent to which an infectious process was responsible for the illnesses is unclear (25).
Climate change could be affecting the ecology of pathogenic fungi in ways that are not yet fully understood; even minor or gradual changes in temperature, moisture, and wind patterns might affect fungal growth, distribution, and dispersal (20). For example, warmer average global temperatures may allow the geographic range of fungi typically restricted to tropical and subtropical environments, such as Cryptococcus gattii, to expand into areas that are currently more temperate (20). Global warming has also been hypothesized to select for fungi with tolerance to warmer temperatures (20). The relative scarcity of fungal diseases among mammals has been hypothesized to be associated with the inability of many fungal species to survive at temperatures >37°C; however, warmer ambient temperatures may enable nonpathogenic fungi to acquire the ability to infect warm-blooded hosts (20).
Huppert and Sparks suggest that global climate change is contributing to greater frequency and severity of extreme weather events and that current patterns of population growth, urbanization, and human activity create conditions that render many communities increasingly vulnerable to these hazards (39). Coupled with an increased risk for natural disasters, a larger or more geographically widespread ecologic burden of pathogenic fungi could lead to greater numbers of disaster-associated fungal infections through any of several mechanisms: inhalation of spores dispersed as a result of geophysical disruption, traumatic implantation of fungi into wounds contaminated with organic matter, or infection associated with suboptimal medical care where the local health care system has been damaged or destroyed. Altogether, a combination of factors including genetic and biological aspects of host–pathogen interactions; changing features of the physical environment; and social, political, or economic influences could lead to the emergence of new fungal pathogens or increased numbers of infections by known pathogens (40).
Disasters are complex events that can result in a wide range of health effects, although infectious disease outbreaks as an immediate consequence of disasters are uncommon. Health care providers should be aware of the possibility for cases or clusters of community-acquired or health care–associated fungal infections among disaster survivors because these infections often appear clinically similar to bacterial infections and can be associated with serious illness and death. These infections can occur in persons who do not have the typical immunocompromising risk factors for fungal infection but who have experienced near-drowning, trauma, or other unusual exposure to the environment, such as a dust storm. A fungal infection should be considered early if a patient has a persistent or progressive infection that is not responding to initial antibacterial treatment, particularly because rapid diagnosis and administration of appropriate antifungal therapy can improve patient outcomes. Prompt restoration of disaster-affected aspects of the local health care infrastructure may help facilitate earlier diagnosis and treatment and possibly reduce the risk for infection associated with the use of contaminated medical equipment or substandard care. Strategies to reduce disaster-associated fungal infections should be considered within the broader context of comprehensive and sustainable risk reduction methods to prevent disaster-related injury and illness.
Ms Benedict is an epidemiologist in the Mycotic Diseases Branch, Centers for Disease Control and Prevention. Her interests include the epidemiology of fungal infections and health communications.
Dr Park is a medical officer in the Mycotic Diseases Branch, Centers for Disease Control and Prevention, and leader of the branch epidemiology team. His research interests include the prevention and epidemiology of fungal infections.
References
- Noji EK. The public health consequences of disasters. Prehosp Disaster Med. 2000;15:147–57 .PubMedGoogle Scholar
- World Health Organization. WHO definitions: emergencies [cited 2013 Aug 12]; http://www.who.int/hac/about/definitions/en/index.html
- Floret N, Viel JF, Mauny F, Hoen B, Piarroux R. Negligible risk for epidemics after geophysical disasters. Emerg Infect Dis. 2006;12:543–8. DOIPubMedGoogle Scholar
- Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo Y-C, Adebanjo T, Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214–25. DOIPubMedGoogle Scholar
- Kawakami Y, Tagami T, Kusakabe T, Kido N, Kawaguchi T, Omura M, Disseminated aspergillosis associated with tsunami lung. Respir Care. 2012;57:1674–8. DOIPubMedGoogle Scholar
- Nakamura Y, Utsumi Y, Suzuki N, Nakajima Y, Murata O, Sasaki N, Multiple Scedosporium apiospermum abscesses in a woman survivor of a tsunami in northeastern Japan: a case report. J Med Case Reports. 2011;5:526. DOIPubMedGoogle Scholar
- Igusa R, Narumi S, Murakami K, Kitawaki Y, Tamii T, Kato M, Escherichia coli pneumonia in combination with fungal sinusitis and meningitis in a tsunami survivor after the Great East Japan Earthquake. Tohoku J Exp Med. 2012;227:179–84. DOIPubMedGoogle Scholar
- Riddel CE, Surovik JG, Chon SY, Wang WL, Cho-Vega JH, Cutlan JE, Fungal foes: presentations of chromoblastomycosis post-hurricane Ike. Cutis. 2011;87:269–72 .PubMedGoogle Scholar
- Rao CY, Reed D, Kemmerly S, Morgan J, Fridkin SK. Assessing invasive mold infections, mold exposures and personal protective equipment use among immunocompromised New Orleans residents after Hurricane Katrina. Society for Healthcare Epidemiology of America 17th Annual Scientific Meeting 2007; 2007 Apr 14–17; Baltimore. [cited 2013 Aug 12]; http://www.shea-online.org/Assets/files/The_Environment.doc.pdf
- Petrini B, Farnebo F, Hedblad MA, Appelgren P. Concomitant late soft tissue infections by Cladophialophora bantiana and Mycobacterium abscessus following tsunami injuries. Med Mycol. 2006;44:189–92. DOIPubMedGoogle Scholar
- Garzoni C, Emonet S, Legout L, Benedict R, Hoffmeyer P, Bernard L, Atypical infections in tsunami survivors. Emerg Infect Dis. 2005;11:1591–3. DOIPubMedGoogle Scholar
- Gunaratne PS, Wijeyaratne CN, Chandrasiri P, Sivakumaran S, Sellahewa K, Perera P, An outbreak of Aspergillus meningitis following spinal anaesthesia for caesarean section in Sri Lanka: a post-tsunami effect? Ceylon Med J. 2006;51:137–42 .PubMedGoogle Scholar
- Andresen D, Donaldson A, Choo L, Knox A, Klaassen M, Ursic C, Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet. 2005;365:876–8. DOIPubMedGoogle Scholar
- Snell BJ, Tavakoli K. Necrotizing fasciitis caused by Apophysomyces elegans complicating soft-tissue and pelvic injuries in a tsunami survivor from Thailand. Plast Reconstr Surg. 2007;119:448–9. DOIPubMedGoogle Scholar
- Maegele M, Gregor S, Yuecel N, Simanski C, Paffrath T, Rixen D, One year ago not business as usual: wound management, infection and psychoemotional control during tertiary medical care following the 2004 tsunami disaster in Southeast Asia. Crit Care. 2006;10:R50. DOIPubMedGoogle Scholar
- Schneider E, Hajjeh RA, Spiegel RA, Jibson RW, Harp EL, Marshall GA, A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA. 1997;277:904–8. DOIPubMedGoogle Scholar
- Patiño JF, Castro D, Valencia A, Morales P. Necrotizing soft tissue lesions after a volcanic cataclysm. World J Surg. 1991;15:240–7. DOIPubMedGoogle Scholar
- Flynn NM, Hoeprich PD, Kawachi MM, Lee KK, Lawrence RM, Goldstein E, An unusual outbreak of windborne coccidioidomycosis. N Engl J Med. 1979;301:358–61. DOIPubMedGoogle Scholar
- Williams PL, Sable DL, Mendez P, Smyth LT. Symptomatic coccidioidomycosis following a severe natural dust storm. An outbreak at the Naval Air Station, Lemoore, Calif. Chest. 1979;76:566–70.
- Garcia-Solache MA, Casadevall A. Global warming will bring new fungal diseases for mammals. MBio. 2010;1:e00061–10. DOIPubMedGoogle Scholar
- Le T, Wolbers M, Chi NH, Quang VM, Chinh NT, Lan NP, Epidemiology, seasonality, and predictors of outcome of AIDS-associated Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis. 2011;52:945–52. DOIPubMedGoogle Scholar
- Tamerius JD, Comrie AC. Coccidioidomycosis incidence in Arizona predicted by seasonal precipitation. PLoS ONE. 2011;6:e21009. DOIPubMedGoogle Scholar
- Park BJ, Pappas PG, Wannemuehler KA, Alexander BD, Anaissie EJ, Andes DR, Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001–2006. Emerg Infect Dis. 2011;17:1855–64. DOIPubMedGoogle Scholar
- Allworth AM. Tsunami lung: a necrotising pneumonia in survivors of the Asian tsunami. Med J Aust. 2005;182:364 .PubMedGoogle Scholar
- Ogawa H, Fujimura M, Takeuchi Y, Makimura K. Chronic cough in a tsunami-affected town. Pulm Pharmacol Ther. 2012;25:11. DOIPubMedGoogle Scholar
- Wang Y, Hao P, Lu B, Yu H, Huang W, Hou H, Causes of infection after earthquake, China, 2008. Emerg Infect Dis. 2010;16:974–5. DOIPubMedGoogle Scholar
- Wang T, Li D, Xie Y, Kang M, Chen Z, Chen H, The microbiological characteristics of patients with crush syndrome after the Wenchuan earthquake. Scand J Infect Dis. 2010;42:479–83. DOIPubMedGoogle Scholar
- Gilbert DN, Sanford JP, Kutscher E, Sanders CV Jr, Luby JP, Barnett JA. Microbiologic study of wound infections in tornado casualties. Arch Environ Health. 1973;26:125–30. DOIPubMedGoogle Scholar
- Pappagianis D, Einstein H. Tempest from Tehachapi takes toll or Coccidioides conveyed aloft and afar. West J Med. 1978;129:527–30 .PubMedGoogle Scholar
- Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;25:896–907 . DOIPubMedGoogle Scholar
- Lim JH, Yoon D, Jung G, Joo Kim W, Lee HC. Medical needs of tsunami disaster refugee camps. Fam Med. 2005;37:422–8 .PubMedGoogle Scholar
- Ivers LC, Ryan ET. Infectious diseases of severe weather-related and flood-related natural disasters. Curr Opin Infect Dis. 2006;19:408–14. DOIPubMedGoogle Scholar
- Hiransuthikul N, Tantisiriwat W, Lertutsahakul K, Vibhagool A, Boonma P. Skin and soft-tissue infections among tsunami survivors in southern Thailand. Clin Infect Dis. 2005;41:e93–6. DOIPubMedGoogle Scholar
- Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–9. DOIPubMedGoogle Scholar
- Hussey SM, Gander R, Southern P, Hoang MP. Subcutaneous phaeohyphomycosis caused by Cladophialophora bantiana. Arch Pathol Lab Med. 2005;129:794–7 .PubMedGoogle Scholar
- Institute of Medicine, Committee on Damp Indoor Spaces and Health. Damp indoor spaces and health. Washington (DC): The National Academies Press; 2004.
- Centers for Disease Control and Prevention. Health concerns associated with mold in water-damaged homes after hurricanes Katrina and Rita–New Orleans area, Louisiana, October 2005. MMWR Morb Mortal Wkly Rep. 2006;55:41–4 .PubMedGoogle Scholar
- Rao CY, Kurukularatne C, Garcia-Diaz JB, Kemmerly SA, Reed D, Fridkin SK, Implications of detecting the mold Syncephalastrum in clinical specimens of New Orleans residents after hurricanes Katrina and Rita. J Occup Environ Med. 2007;49:411–6. DOIPubMedGoogle Scholar
- Huppert HE, Sparks RS. Extreme natural hazards: population growth, globalization and environmental change. Philos Transact Ser A Math Phys. Eng Sci. 2006;364:1875–88.
- Warnock DW. Fungal diseases: an evolving public health challenge. Med Mycol. 2006;44:697–705. DOIPubMedGoogle Scholar
Figure
Table
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Invasive Fungal Infections after Natural Disasters
CME Questions
1. You are responding to provide medical care in a location that was devastated by an earthquake and subsequent tsunami 2 days ago. What should you consider regarding fungal infections in this situation?
A. Most fungi are human pathogens
B. Disasters pose a higher risk for fungal infections, but smaller activities such as excavations do not
C. Coccidioides spp. are endemic to the southwestern United States
D. Most cases of coccidioidomycosis are promptly identified and treated appropriately
2. You treat multiple individuals who had prolonged exposure to water and nearly drowned. Which of the following statements regarding waterborne fungal infections is most accurate?
A. “Tsunami lung” refers specifically to fungal infections
B. Pseudallescheria boydii is thought to be the most common fungal pathogen associated with near-drowning
C. After the 2004 Indian Ocean tsunami, most acute respiratory illness was the result of influenza, not fungal infections
D. Aspergillus spp. generally have no role in post-disaster infections
3. What should you consider regarding soft tissue infections after a natural disaster?
A. Most soft tissue infections after disasters are the result of fungi
B. Fungal soft tissue infections generally appear similar to bacterial infections at the initial presentation
C. Mucormycosis results in death in fewer than 3% of cases
D. Surgical debridement is usually contraindicated in cases of mucormycosis
4. Two weeks after the disaster, a number of patients are concerned about mold growing in their shelter. What can you tell them?
A. Indoor mold exposure can lead to cough and wheeze
B. Mold exposure frequently leads to infection, even among immunocompetent hosts
C. Hurricane Katrina was associated with high rates of mold infection among immunocompromised individuals
D. Molds generally result in invasive infection but not colonization
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
Related Links
Table of Contents – Volume 20, Number 3—March 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Benjamin J. Park, Centers for Disease Control and Prevention,1600 Clifton Rd NE, Mailstop C09, Atlanta, GA 30033, USABenjamin J. Park, Centers for Disease Control and Prevention,1600 Clifton Rd NE, Mailstop C09, Atlanta, GA 30033, USABenjamin J. Park, Centers for Disease Control and Prevention,1600 Clifton Rd NE, Mailstop C09, Atlanta, GA 30033, USA
Top