Volume 20, Number 6—June 2014
Dispatch
Identification of Possible Virulence Marker from Campylobacter jejuni Isolates
Abstract
A novel protein translocation system, the type-6 secretion system (T6SS), may play a role in virulence of Campylobacter jejuni. We investigated 181 C. jejuni isolates from humans, chickens, and environmental sources in Vietnam, Thailand, Pakistan, and the United Kingdom for T6SS. The marker was most prevalent in human and chicken isolates from Vietnam.
Campylobacter species are the principal bacterial cause of human foodborne enterocolitis worldwide (1). Despite the global significance of C. jejuni as a leading cause of diarrheal disease (2), the mechanisms of pathogenesis of C. jejuni are not well understood. Research on Campylobacter epidemiology has largely been conducted in high-income countries and therefore may not be representative of global patterns.
Recently, a novel class of protein translocation system was identified in gram-negative bacteria. This system, named the type-6 secretion system (T6SS), has been found to play roles in pathogen–pathogen and host–pathogen interactions and has a major effect on virulence in a range of pathogens, including Vibrio cholerae (3–6) (reviewed in 7,8). A functional T6SS was recently identified in C. jejuni (9,10) and found to have several roles in virulence, influencing cell adhesion, cytotoxicity toward erythrocytes, and colonization of mice (9,10). However, it is unknown whether T6SS changes the effects of these pathogens in human infection.
In this study, we aimed to determine whether presence of T6SS in C. jejuni is potentially a marker associated with more severe human disease. Moreover, because human infection with C. jejuni is often associated with contact with poultry, we investigated whether poultry harbor C. jejuni that possess T6SS.
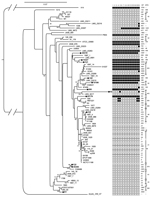
To partially address bias toward study of C. jejuni strains from high-income countries and the under-representation of strains from Asia in previous studies, we previously sequenced the genomes of 12 clinical isolates of C. jejuni from Asia: 4 from Thailand, 3 from Pakistan, and 5 from Vietnam (J. Harrison, unpub. data; Figure 1). We noted that 8 (67%) of these isolates possessed a cluster of genes homologous to the recently described T6SS (Figure 1). This finding was in contrast to findings regarding previously sequenced C. jejuni genomes; only 10 (14%) of 71 previously sequenced C. jejuni strains possessed an apparently intact T6SS gene cluster (Figure 1; full listing of genomes is in Technical Appendix Table 1). Several other strains from our study and previously sequenced strains contained >1 T6SS genes but not a complete T6SS cluster. Figure 1 shows the presence and absence of each T6SS gene in each available genome sequence (J. Harrison, unpub. data) and the previously sequenced strains. A nonrandom distribution of T6SS can be seen across the phylogenetic diversity of C. jejuni; T6SS is limited to certain clades, and degeneration of the T6SS gene cluster apparently occurs in parallel within several of those clades (Figure 1).
Our genome sequencing analysis indicated that strains possessing a complete T6SS cluster could be distinguished by the presence of the hcp gene (Figure 1) (9,10). Therefore, we used hcp as a proxy for determining the presence of a functional T6SS in 181 C. jejuni isolates from chickens, humans, and environmental sources (collections of the Oxford University Clinical Research Unit and the University of Exeter; online Technical Appendix Table 2). We designed and used a multiplex PCR (Technical Appendix Table 3) to screen for the presence of hcp in these isolates; the conserved C. jejuni housekeeping gene, gltA, was used as a positive control.
Of the 181 isolates, 28 originated from chickens in the United Kingdom and 21 from chickens in Vietnam. The hcp gene was found significantly more often in isolates from Vietnam (15 [71.4%] isolates) than in those from the United Kingdom (1 [3.5%] isolate) (p<0.01 by 2-sample Z-test; Technical Appendix Figure 1). An additional 38 of the isolates were from humans in the United Kingdom and 33 from humans in Vietnam; again, the hcp gene was significantly more prevalent in isolates from Vietnam (20 [60.6%] isolates) than those from the United Kingdom (1 [2.6%] isolate) (p<0.01 by 2-sample Z-test; Technical Appendix Figure 2).
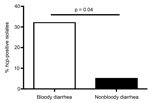
We also found that patients infected with hcp-positive C. jejuni experienced bloody diarrhea more commonly than those infected with hcp-negative C. jejuni. For the 36 isolates for which detailed clinical data on patients were available, 6 (31.6%) of 19 patients in Vietnam who were infected with hcp-positive C. jejuni had bloody diarrhea, compared with 1 (5.9%) of 17 patients infected with hcp-negative C. jejuni (p<0.05 by 2-sample Z-test) (Figure 2). These results suggest a potential correlation between T6SS and bloody diarrhea, a serious clinical manifestation of the infection that results in higher rates of hospitalization and greater need for treatment with antimicrobial drugs (11). Moreover, Campylobacter-related septicemia developed in the 1 patient in the United Kingdom who was infected with a T6SS-positive strain (11). These data suggest that infection with the C. jejuni T6SS genotypic strains is associated with more severe disease. However, for sample bias to be ruled out, a comprehensive study is required in which the prevalence of T6SS is measured in C. jejuni samples from patients with mild and severe forms of infection.
We found a number of C. jejuni strains from humans and poultry that possessed the T6SS cluster, although some strains showed a slightly modified gene order (Technical Appendix Table 1 and Figure 3). However, most (61 [85.9%] of 71) of the previously sequenced C. jejuni isolates lacked a complete T6SS gene cluster (Figure 1); this finding might explain why T6SS was not discovered in C. jeuni sooner. Conversely, our PCR-based study frequently identified the hcp marker in isolates from Thailand, Pakistan, and Vietnam (Table). We cannot be certain that all of the isolates with the hcp marker possessed a complete and functional T6SS gene cluster, but the hcp gene is consistently associated with the presence of a complete T6SS cluster in all available sequenced C. jejuni genomes (Figure 1). This correlation lends confidence to the use of hcp as a proxy.
Poultry are a well-documented reservoir of human Campylobacter infection (12). We found that Campylobacter strains harboring the hcp marker were significantly associated with chickens in Asia. Large numbers of poultry are imported into North America and Europe from low-income countries, including Thailand (13). This process could introduce T6SS-positive Campylobacter genotypes into the food chains of the importing countries, posing a potential emerging threat to public health.
Our results suggest that the T6SS may be more prevalent in C. jejuni in Vietnam, Pakistan, and Thailand than in the United Kingdom. Furthermore, our results suggest that hcp may be a marker associated with severe human disease caused by C. jejuni infection in Vietnam, although there is no evidence that the association is causal. Chickens imported from these countries could be a source of hcp-positive strains and may have the potential to cause severe human infection.
Mr Harrison is a PhD student at the University of Exeter under the supervision of D.S. His research focuses on using bioinformatic methods to investigate the comparative genomics of emerging diseases and plant-associated microbes.
Acknowledgments
We thank Konrad Paszkiewicz and Karen Moore for assistance with whole-genome sequencing.
The work was partly supported by the UK Biotechnology and Biological Sciences Research Council, award BB/1024631/1 to R.T., D.S., and O.C.; by a Wellcome Trust Institutional Strategic Support Award (WT097835MF); and by a studentship awarded to J.H.
References
- Adak GK, Meakins SM, Yip H, Lopman BA, O’Brien SJ. Disease risks from foods, England and Wales, 1996–2000. Emerg Infect Dis. 2005;11:365–72 . DOIPubMedGoogle Scholar
- Allos BM. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis. 2001;32:1201–6. DOIPubMedGoogle Scholar
- Das S, Chakrabortty A, Banerjee R, Roychoudhury S, Chaudhuri K. Comparison of global transcription responses allows identification of Vibrio cholerae genes differentially expressed following infection. FEMS Microbiol Lett. 2000;190:87–91. DOIPubMedGoogle Scholar
- Ishikawa T, Sabharwal D, Bröms J, Milton DL, Sjöstedt A, Uhlin BE, Pathoadaptive conditional regulation of the type VI secretion system in Vibrio cholerae O1 strains. Infect Immun. 2012;80:575–84. DOIPubMedGoogle Scholar
- Parsons DA, Heffron F. sciS, an icmF homolog in Salmonella enterica serovar Typhimurium, limits intracellular replication and decreases virulence. Infect Immun. 2005;73:4338–45. DOIPubMedGoogle Scholar
- Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A. 2006;103:1528–33. DOIPubMedGoogle Scholar
- Mulder DT, Cooper CA, Coombes BK. Type VI secretion system-associated gene clusters contribute to pathogenesis of Salmonella enterica serovar Typhimurium. Infect Immun. 2012;80:1996–2007. DOIPubMedGoogle Scholar
- Lertpiriyapong K, Gamazon ER, Feng Y, Park DS, Pang J, Botka G, Campylobacter jejuni type VI secretion system: roles in adaptation to deoxycholic acid, host cell adherence, invasion, and in vivo colonization. PLoS ONE. 2012;7:e42842. DOIPubMedGoogle Scholar
- Bleumink-Pluym NMC, van Alphen LB, Bouwman LI, Wösten MMSM, van Putten JPM. Identification of a functional type VI secretion system in Campylobacter jejuni conferring capsule polysaccharide sensitive cytotoxicity. PLoS Pathog. 2013;9:e1003393. DOIPubMedGoogle Scholar
- Kuşkonmaz B, Yurdakök K, Yalçin SS, Ozmert E. Comparison of acute bloody and watery diarrhea: a case control study. Turk J Pediatr. 2009;51:133–40 .PubMedGoogle Scholar
- Harris NV, Weiss NS, Nolan CM. The role of poultry and meats in the etiology of Campylobacter jejuni/coli enteritis. Am J Public Health. 1986;76:407–11. DOIPubMedGoogle Scholar
- Food and Agriculture Organization of the United Nations. Agribusiness handbook: poultry meat and eggs. 2010 [cited 2013 Apr 1]. http://www.fao.org/fileadmin/user_upload/tci/docs/1_AH9-Poultry%20Meat%20&%20Eggs.pdf
Figures
Table
Cite This ArticleTable of Contents – Volume 20, Number 6—June 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Olivia L. Champion, University of Exeter, Geoffrey Pope Building, Stocker Road, Exeter EX4 4QD, UK
Top