Volume 20, Number 8—August 2014
Dispatch
Borrelia crocidurae Infection in Acutely Febrile Patients, Senegal
Abstract
As malaria cases in Africa decline, other causes of acute febrile illness are being explored. To determine incidence of Borrelia crocidurae infection during June 2010–October 2011, we collected 1,566 blood specimens from febrile patients in Senegal. Incidence was high (7.3%). New treatment strategies, possibly doxycycline, might be indicated for febrile patients.
As malaria cases decline, the need for programs to study the roles of other causes of febrile syndromes in Africa increases (1). Other causes of fever in outpatients include typhoid and paratyphoid fevers; pneumococcal bacteremia; and a spectrum of viral infections, including influenza, yellow fever, dengue, chikungunya, and Rift Valley fever (2). In preliminary studies, we detected the DNA of Borrelia crocidurae, Rickettsia spp., Tropheryma whipplei, and Coxiella burnetii in environmental samples and blood specimens from febrile patients in Senegal (3–6).
In western Africa, tick-borne relapsing fever (TBRF) is caused by B. crocidurae; this acute febrile illness produces multiple recurrences of nonspecific signs and symptoms, including fever, headache, myalgia, and arthralgia. After decades of neglect, TBRF has again been detected (7) and is thought to be one of the major causes of fever in Africa. The reported incidence rate for TBRF in western Africa is high, reaching 25 cases per 100 person-years or accounting for 13% of febrile illnesses treated at rural dispensaries (4,8). This incidence rate is even higher than that for TBRF caused by B. duttonii in eastern Africa (9). A study from Tanzania found B. duttonii DNA in 3.9% of blood samples (10).
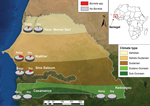
In 2008, we began to create a network of rural dispensaries from which to recruit patients (Table; Figure 1). The 5 study sites covered several ecosystems, ranging from dry Sahelian in northern Senegal (Keur Momar Sarr, Niakhar, and Sine-Saloum) to humid sub-Guinean in southern Senegal (Casamance and Kedougou). Two seasons are typical: dry (November–May) and rainy (June–October). The National Ethics Committee of Senegal approved the study (11,12). Since 2010, the populations of both villages in Sine-Saloum (Dielmo and Ndiop) have benefited from routine rapid point-of-care laboratory diagnostics (12).
From June 2010 through October 2011, fingerstick blood samples (200 μL) were collected from 1,549 febrile patients (axillary temperature >37.5°C) at 14 dispensaries and 91 randomly selected healthy villagers in Senegal. Samples were subjected to DNA extraction. Digesting, binding, and washing were performed directly in the village dispensaries by use of the QIAamp kit (QIAGEN, Hilden, Germany) as previously reported (6). DNA elution was performed at Aix-Marseille Université in Marseille, France. Quantitative PCR (qPCR) was performed by using primers and probes specific for the genus Borrelia. All samples positive for Borrelia spp. were subjected to standard PCR (flaB gene) (4). To determine the quality of the extracted DNA, we also measured the human actin gene (5). The samples were considered positive only if qPCR and flaB-based PCR results were positive; the sequencing of all flaB amplicons demonstrated that they belonged to B. crocidurae.
Isolation of borreliae involved intraperitoneal inoculation of laboratory BALB/c mice with 100 μL of patient capillary blood. Borreliae in mice were detected by microscopic examination of Giemsa-stained peripheral blood smears followed by qPCR of blood samples.
The sex ratio for the 1,549 patients did not differ significantly among sites (772 male and 777 female patients). An analysis of 6 age groups (<12 months, 1–3 years, 4–6 years, 7–15 years, 16–29 years, and >30 years) showed no differences among sites. All tested samples from clinically healthy persons had negative qPCR results for borreliae.
The incidence rate was calculated as the number of febrile episodes divided by the person-time multiplied by 1,000 (data available only for the Sine-Saloum site). The incidence rate of febrile episodes was 0.80 in Dielmo and 0.36 in Ndiop (p<0.05). Among the 1,566 samples tested, 115 (7.3%) were positive for B. crocidurae. The incidence rate for TBRF was 9.7 cases/100 persons in Dielmo and 2.4 cases/100 persons in Ndiop. The first autochthonous cases in Ndiop, which was previously considered borreliosis free, were observed in October 2010; incidence was significantly lower in Ndiop than in Dielmo (p<0.05). All cases registered in Ndiop before October 2010 were included in the epidemiologic investigation and considered to be imported. The proportion of the Borrelia-positive samples was significantly higher for northern sites with a drier Sudanian climate; positivity reached 19.1% (33/173) in Niakhar (Table). By analyzing the epidemiologic questionnaires completed by families of ill persons, we determined that the 2 TBRF cases in Casamance were imported from the northern regions of Senegal by seasonal workers.
Patients most frequently infected were 7–15 years of age (13.5%, 43/318), unlike in eastern Africa where younger persons are more frequently infected (9). No positive results were found among the 155 children <12 months of age, but positive results were found for 16 (4.8%) of the 352 children 1–3 years of age (p<0.05). Unlike in other northern regions, in Sine-Saloum, the proportion of Borrelia-positive samples was significantly higher for samples collected during the dry (16.9%, 40/237) than the rainy season (6.9%, 30/432); p<0.0001 (Figure 1).
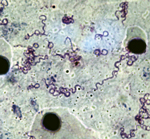
We identified 20 patients (49 samples) for whom 2–4 samples were positive for B. crocidurae. The interval between the sample collections was short (5–30 days) for 13 persons, average (30–66 days) for 4 persons, and long (102–381 days) for 3 persons. Two Borrelia isolates (no. 03–02 from Ndiop and no. 19/31 from Dielmo) were recovered from the peripheral blood of 2 febrile patients. The bacteria had a morphologic appearance that was typical for borreliae (Figure 2). A BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) search for the sequenced flaB gene (JX119098) demonstrated that the isolates were nearly identical with the type Achema strain of B. crocidurae (CP003426).
We detected an alarmingly high proportion of Borrelia DNA in the blood of febrile patients in Senegal. The presence of this DNA is strongly and specifically linked to the fever because no Borrelia DNA was identified among the 90 control participants. In Tanzania, however, borreliae have been identified in up to 33% of blood samples obtained from asymptomatic blood donors who lived in similar conditions as ill persons (13).
The geographic repartition of TBRF is linked to drier climates (9). We observed autochthonous cases only in northern Senegal, roughly north of the 13°30′ parallel. We noted the recent extension of B. crocidurae into the village of Ndiop, which had been free of B. crocidurae. This extension might be linked to recent climate changes (14). The person-year incidence of borreliosis in our study (6.1 cases/100 population) is similar to that reported by Vial et al. (4 cases/100 population) for the interepidemic period (8).
We report a unique series of cases in which Borrelia DNA was identified several times consecutively in the blood of the same patient. For 17 patients for whom the time between positive samples was short or average (up to 66 days), repeated detection of Borrelia DNA during repeated episodes of fever could be explained by relapses. However, reinfection is strongly suspected in 3 patients because the interval between 2 positive samples was >100 days. To the best of our knowledge, reinfection with relapsing fever borreliae has not been previously reported in Africa. The phenomenon of easy reinfection after treatment with tetracycline has been reported for the relapsing-fever group B. hermsii in vervet monkeys, which could be reinfected 12–36 weeks after primary infection (15).
In conclusion, the incidence of TBRF and the proportion of borreliosis cases among febrile patients in Senegal is very high and, in at least 1 region (Niakhar), exceeds that of malaria. This considerably high incidence rate should lead to the development of new therapeutic strategies that could be based on treating febrile patients in Senegal with doxycycline.
Dr Mediannikov is a researcher in epidemiology and microbiology in Dakar, Senegal. His scientific interests include vectorborne diseases and other causes of nonmalarial fever in Africa.
Acknowledgment
We thank the Agence National de Recherche, grant MALEMAF (Research of Emergent Pathogens in Africa) and La Fondation Méditerranée Infection for financial support; all villagers who participated in the study; Annick Bernard, Aliou Diallo, Denis Piak, and Khadim Mbacke Leye for technical support; and Pascal Weber for photography.
References
- Gwer S, Newton CR, Berkley JA. Over-diagnosis and co-morbidity of severe malaria in African children: a guide for clinicians. Am J Trop Med Hyg. 2007;77(Suppl):6–13 .PubMedGoogle Scholar
- Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412 . DOIPubMedGoogle Scholar
- Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Bassene H, Molez JF, Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis. 2010;4:e654 . DOIPubMedGoogle Scholar
- Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis. 2011;17:883–5. DOIPubMedGoogle Scholar
- Fenollar F, Mediannikov O, Socolovschi C, Bassene H, Diatta G, Richet H, Tropheryma whipplei bacteremia during fever in rural West Africa. Clin Infect Dis. 2010;51:515–21 . DOIPubMedGoogle Scholar
- Mediannikov O, Diatta G, Fenollar F, Sokhna C, Trape JF, Raoult D. Tick-borne rickettsioses, neglected emerging diseases in rural Senegal. PLoS Negl Trop Dis. 2010;4:e821. DOIPubMedGoogle Scholar
- Bergeret C, Raoult A. Notes sur les formes nerveuses de la fièvre récurrente-–fièvre récurrente à tiques en Afrique Occidentale Française. Bul Med Afrique Occidentale Française. 1934;27:593–8.
- Vial L, Diatta G, Tall A. Ba el H, Bouganali H, Durand P, et al. Incidence of tick-borne relapsing fever in West Africa: longitudinal study. Lancet. 2006;368:37–43.
- Cutler SJ. Possibilities for relapsing fever reemergence. Emerg Infect Dis. 2006;12:369–74 . DOIPubMedGoogle Scholar
- Reller ME, Clemens EG, Schachterle SE, Mtove GA, Sullivan DJ, Dumler JS. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. J Clin Microbiol. 2011;49:3245–9. DOIPubMedGoogle Scholar
- Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–37 .PubMedGoogle Scholar
- Sokhna C, Mediannikov O, Fenollar F, Bassene H, Diatta G, Tall A, Point-of-care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl Trop Dis. 2013;7: e1999. .DOIGoogle Scholar
- Cutler SJ, Bonilla EM, Singh RJ. Population structure of East African relapsing fever Borrelia spp. Emerg Infect Dis. 2010;16:1076–80. DOIPubMedGoogle Scholar
- Trape JF, Godeluck B, Diatta G, Rogier C, Legros F, Albergel J, The spread of tick-borne borreliosis in West Africa and its relationship to sub-Saharan drought. Am J Trop Med Hyg. 1996;54:289–93 .PubMedGoogle Scholar
- Felsenfeld O, Wolf RH. Reinfection of vervet monkeys (Cercopithecus aethiops) with Borrelia hermsii. Res Commun Chem Pathol Pharmacol. 1975;11:147–50 .PubMedGoogle Scholar
Figures
Table
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 20, Number 8—August 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Didier Raoult, URMITE, IRD 198, UM63, CNRS 7278, INSERM 1095, Aix-Marseille Université, 13005 Marseille, France
Top