Volume 20, Number 8—August 2014
Dispatch
Novel Reassortant Influenza A(H5N8) Viruses in Domestic Ducks, Eastern China
Abstract
Domestic ducks are natural reservoirs of avian influenza viruses and serve as reassortant hosts for new virus subtypes. We isolated 2 novel influenza A(H5N8) viruses from domestic ducks in eastern China, sequenced their genomes, and tested their pathogenicity in chickens and mice. Circulation of these viruses may pose health risks for humans.
Avian influenza viruses are members of the family Orthomyxoviridae and contain 8 segments of single-stranded RNA with negative polarity (1). These viruses are classified into subtypes on the basis of their envelope proteins hemagglutinin (HA) and neuraminidase (NA). Aquatic birds, including domestic ducks, have been considered the natural reservoir of these viruses (2). Although domestic ducks do not usually display symptoms when they are infected with these viruses, they provide an environment for the reassortment of low pathogenicity avian influenza viruses, which can serve as progenitors of highly pathogenic avian influenza viruses (3).
Because live poultry markets are considered a major source of avian influenza virus dissemination and sites for potential influenza virus reassortment, as well as interspecies transfer (3,4), we participated in active surveillance of these virus in live poultry markets. We sequenced genes from 2 novel influenza A(H5N8) viruses isolated from domestic ducks in eastern China and evaluated their pathogenicity in chickens and mice.
During surveillance of poultry for avian influenza viruses in live poultry markets in Zhejiang Province in eastern China in 2013, we isolated 2 influenza A(H5N8) viruses, A/duck/Zhejiang/W24/2013(H5N8) (W24) and A/duck/Zhejiang/6D18/2013(H5N8) (6D18), from domestic ducks. To better understand genetic relatedness between these viruses, we sequenced all gene segments of these 2 viruses and compared them with influenza virus sequences in GenBank. We also determined the virulence of the 2 isolates in chickens and mice.
For virus isolation, cloacal swab specimens from domestic ducks were inoculated into embryonated chicken eggs as described (5). All experiments with viruses were performed in a Biosafety Level 3 laboratory.
RNA was extracted by using the Viral RNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. All gene segments were amplified with primers, and fragments were sequenced and analyzed as described (6–8). Sequence data obtained were submitted to GenBank under accession nos. KJ476663–KJ476678.
Sequence analysis showed that all sequences of 8 genes (HA, NA, basic polymerase 1, basic polymerase 2 [PB2], acidic polymerase, nucleoprotein, matrix protein, and nonstructural protein [NS]) of viruses W24 and 6D18 showed 99.9%–100% sequence similarity (Figure 1). Results show that the HA gene of W24 was closely related to those HA genes of H5N8 subtype viruses circulating in South Korea in 2014 (9); W24 belongs to clade 2.3.4.
Sequence analysis suggested that these H5N8 subtype viruses were most closely related to isolates from poultry in countries in eastern Asia. Previous studies have shown that H5 subtype viruses within clade 2.3.2 have been circulating widely in poultry and wild birds in China since 2007 (7,10). Our results indicated that the 2 novel H5 subytpe viruses belong to clade 2.3.4, the prevalent lineage in southern China since 2005 (11); thus showing their presence in eastern China. NA gene phylogeny indicated that a novel influenza A(H10N8) virus, which infected humans, had different ancestors for this gene (Figure 1, panel B).Gene phylogenies for 6 other genes indicated that H9N2 subtype viruses circulating in China were not donors of these genes for W24.
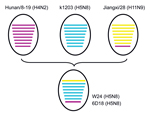
Lee et al. (9) recently reported that that HA and NA genes of 3 H5N8 subtype viruses isolated in South Korea in 2014 had high nucleotide identities with A/duck/Jiangsu/k1203/2010(H5N8). All 8 genes of W24 were closely related to those of H5N8 subtype viruses, such as A/breeder duck/ Korea/Gochang1/2014(H5N8), which are circulating in South Korea. Basic polymerase 1, acidic polymerase, HA, nucleoprotein, NA, and matrix genes of W24 were also closely related to those of A/duck/Jiangsu/k1203/2010(H5N8). PB2 and NS genes of W24 were most closely related to those of A/environment/Jiangxi/28/2009(H11N9) and A/duck/Hunan/8–19/2009(H4N2), respectively (Table 1; Figure 2). On the basis of analysis of phylogenetic relationships, we found that W24 was a reassortant virus that derived its genes from a virus of a different subtype from poultry in China. We also found that H5N8 subtype viruses had been present in eastern China for several years and these viruses might have been spread to other countries by wild birds in recent years.
On the basis of deduced amino acid sequences of HA genes, we found that the HA cleavage site pattern (PLREKRRKR) of the 2 novel H5N8 subtype viruses indicated that these viruses were highly pathogenic. In this study, amino acid sequences of these H5N8 subtype viruses at positions 236–241 and 146–150 were NGQRGR and GVSAA, respectively. Receptor-binding sites (Gln226 and Gly228) of H5N8 subtype viruses were similar to those of the 2 novel H5N8 subtype viruses, which suggested that these 2 viruses would preferentially bind to avian-like receptors (7). The PB2 protein Lys627Glu mutation has been reported to influence the host range and confer increased virulence for H5N1 subtype viruses in animal models (12). This mutation was not observed in PB2 of the 2 novel H5N8 subtype viruses analyzed in this study, which indicated that these 2 viruses had low levels of pathogenicity for mice.
Deletion of several amino acids (position 80–84) in NS1 proteins had been observed more frequently in H5N1 subtype viruses, which indicated possible adaptation of these viruses to avian species (13). This deletion was not observed in the 2 novel H5N8 subtype viruses. These 2 viruses contained the NS1 Pro42Ser mutation, which is associated with increased virulence in mice (14).
To evaluate pathogenicity of W24 and 6D18 in chickens, we inoculated groups of ten 6-week-old specific pathogen–free chickens intravenously with a 106 median egg infective dose of each virus in a 0.2-mL volume of phosphate-buffered saline; deaths were observed over a 10-day period (7). Animal studies were conducted according to the recommendation of the World Organisation for Animal Health (Paris, France). Characteristics of W24 and 6D18 viruses are shown in Table 2. Results showed that these viruses were highly pathogenic to chickens.
To determine the pathogenicity of these viruses in a mammalian host, we inoculated BALB/c mice intranasally with a 106 median egg infective dose for each virus. Over a 14-day period, we observed virus replication in various organs and deaths. After intranasal administration of W24 and 6D18, we observed 5 mice per group for survival over a 14-day period. On day 9 postinfection, high titers of virus was detected in lung and liver but were not detected in brain and heart. Mice had signs of illness but had survival rates of 80% (4/5) and 100% (5/5), respectively, for each virus during 14 days postinfection (Table 2). These results suggested that the 2 novel H5N8 subtype viruses did not kill mice but that they could replicate in the lung. Results of our study are consistent with those of Zhao et al. (15), who reported that H5N8 subtype virus from chickens did not cause deaths in mice.
We isolated 2 influenza A(H5N8) viruses were isolated from domestic ducks in eastern China in 2013. Results of phylogenetic analysis showed that these viruses were reassortant viruses that derived their genes from different virus subtypes. These reassortant H5N8 subtype viruses and their 3 possible parent viruses, A/duck/Jiangsu/k1203/2010 (H5N8), A/environment/Jiangxi/28/2009 (H11N9), and A/duck/Hunan/8–19/2009 (H4N2), were isolated in China. Both H5N8 subtype isolates were highly pathogenic for chickens and showed moderate pathogenicity for mice. Domestic ducks are considered the natural reservoir of avian influenza viruses and serve as reassortant hosts for creation of new virus subtypes. Continued circulation of these viruses may pose health threats for birds and humans.
Acknowledgments
This study was supported by grants from the National Key Technologies Research and Development Program for the 12th Five-Year Plan of China (2012ZX1000-004-005) and the State Key Laboratory of independent task (No.2010ZZ04).
Dr Haibo Wu is a faculty member at the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, School of Medicine, Zhejiang University. His primary research interest is the epidemiology and pathogenic mechanism of avian influenza viruses.
References
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–79 .PubMedGoogle Scholar
- Kawaoka Y, Chambers TM, Sladen WL, Webster RG. Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology. 1988;163:247–50 . DOIPubMedGoogle Scholar
- Liu M, He S, Walker D, Zhou N, Perez DR, Mo B, The influenza virus gene pool in a poultry market in South central china. Virology. 2003;305:267–75 . DOIPubMedGoogle Scholar
- Cardona C, Yee K, Carpenter T. Are live bird markets reservoirs of avian influenza? Poult Sci. 2009;88:856–9 . DOIPubMedGoogle Scholar
- Wu HB, Guo CT, Lu RF, Xu LH, Wo EK, You JB, Genetic characterization of subtype H1 avian influenza viruses isolated from live poultry markets in Zhejiang Province, China, in 2011. Virus Genes. 2012;44:441–9 . DOIPubMedGoogle Scholar
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–89 . DOIPubMedGoogle Scholar
- Hai-bo W, Chao-tan G, Ru-feng L, Li-hua X, En-kang W, Jin-biao Y, Characterization of a highly pathogenic H5N1 avian influenza virus isolated from ducks in Eastern China in 2011. Arch Virol. 2012;157:1131–6 . DOIPubMedGoogle Scholar
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9 . DOIPubMedGoogle Scholar
- Lee Y-J, Kang H-M, Lee E-K, Song B-M, Jeong J, Kwon Y-K, Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg Infect Dis. 2014;20:1086–9. DOIGoogle Scholar
- Hu X, Liu D, Wang M, Yang L, Zhu Q, Li L, Clade 2.3.2 avian influenza virus (H5N1), Qinghai Lake region, China, 2009–2010. Emerg Infect Dis. 2011;17:560–2 . DOIPubMedGoogle Scholar
- Smith GJ, Fan XH, Wang J, Li KS, Qin K, Zhang JX, Emergence and predominance of an H5N1 influenza variant in China. Proc Natl Acad Sci U S A. 2006;103:16936–41 . DOIPubMedGoogle Scholar
- Shinya K, Hamm S, Hatta M, Ito H, Ito T, Kawaoka Y. PB2 amino acid at position 627 affects replicative efficiency, but not cell tropism, of Hong Kong H5N1 influenza A viruses in mice. Virology. 2004;320:258–66 . DOIPubMedGoogle Scholar
- Zhu Q, Yang H, Chen W, Cao W, Zhong G, Jiao P, A naturally occurring deletion in its NS gene contributes to the attenuation of an H5N1 swine influenza virus in chickens. J Virol. 2008;82:220–8 . DOIPubMedGoogle Scholar
- Jiao P, Tian G, Li Y, Deng G, Jiang Y, Liu C, A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J Virol. 2008;82:1146–54 . DOIPubMedGoogle Scholar
- Zhao K, Gu M, Zhong L, Duan Z, Zhang Y, Zhu Y, Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet Microbiol. 2013;163:351–7 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 20, Number 8—August 2014
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Nanping Wu, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, School of Medicine, Zhejiang University, 79 Qingchun Rd, Hangzhou, Zhejiang 310003, China
Top