Volume 20, Number 8—August 2014
Dispatch
Antibodies against MERS Coronavirus in Dromedary Camels, Kenya, 1992–2013
Abstract
Dromedary camels are a putative source for human infections with Middle East respiratory syndrome coronavirus. We showed that camels sampled in different regions in Kenya during 1992–2013 have antibodies against this virus. High densities of camel populations correlated with increased seropositivity and might be a factor in predicting long-term virus maintenance.
Middle East respiratory syndrome coronavirus (MERS-CoV) was discovered in a patient from Saudi Arabia in 2012 and has since caused ≥250 human infections and 93 deaths (1). The evolutionary origins of MERS-CoV and related viral species belonging to the genus Betacoronavirus clade C were attributed to insectivorous bats in Europe and Africa (2–4). Seroprevalence studies of livestock from diverse species showed that dromedary camels from Oman, Saudi Arabia, the United Arab Emirates, Jordan, Qatar, Spain, and Egypt harbored antibodies against MERS-CoV antigens (5–8). Direct evidence for MERS-CoV infection in camels has been found in Qatar, Saudi Arabia, and Egypt. Close similarity of camel-associated and human-associated MERS-CoV sequences suggests that camels are sources of infection for humans and might constitute a zoonotic animal reservoir (5,9,10). Where and when the putative introduction of MERS-CoV into camel populations took place and how the virus is maintained in camel populations remains obscure.
Most livestock camels slaughtered in the Arabian Peninsula and in Egypt are imported from the Greater Horn of Africa, in particular Ethiopia, Somalia, Sudan, and Kenya (11,12). We investigated MERS-CoV antibody levels and distribution patterns in farmed and nomadic camels from Kenya.
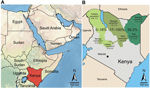
Samples were obtained from 774 dromedary camels in 3 regions in Kenya (Northeastern, Eastern, and Rift Valley [former administrative provinces]) and 7 counties (Mandera, Wajir, Isiolo, Marsabit, Laikipia, Turkana, and Baringo) during 1992–2013 (Figure). Blood samples were obtained from farmed or nomadic camels by jugular vein puncture. Serum samples originated from the archives of the International Livestock and Research Institute (ILRI) (Nairobi, Kenya). Ethical clearance for collection was part of the agreement between the Government of Kenya and ILRI, which provided ILRI with approval to broadly investigate livestock disease in Kenya.
All serum samples were tested for MERS-CoV antibodies by using a recombinant MERS-CoV spike protein subunit 1–based ELISA (rELISA) as described (13). Serum samples were used at a 1:100 dilution, which had been shown to be optimal for screening (13). A positive serum sample from recent studies (6,13) was used as a reference in all experiments. We used the assay-specific cutoff (optical density ratio 0.3) that had been validated in a previous study of camel serum samples (13). A total of 228 (29.5%) of 774 dromedary camels were rated MERS-CoV positive by the rELISA (Table 1). All 228 rELISA-positive serum samples from these 228 camels were subsequently tested at a 1:40 dilution by using an established recombinant immunofluorescence assay and Vero cells expressing MERS-CoV spike protein (6). This confirmatory assay showed that 213 (93.4%) of 228 rELISA-positive serum samples had MERS-CoV antibodies (Table 1).
As a final step, antibody specificity was confirmed by using a highly specific MERS-CoV microneutralization assay as described (6). All 228 rELISA-positive serum samples were tested at a starting dilution of 1:80 and an ending dilution of 1:800 to identify animals with high neutralization titers. A total of 119 (52.2%) 228 rELISA-positive serum samples had MERS-CoV neutralizing antibody titers (range 1:80–1:800) and 14 (6.1%) of 228 had high (>1:800) titers. The highly reactive camel serum samples originated from 3 counties (Wajir, Mandera, and Marsabit) in 2 regions (Northeastern and Eastern). The highest determined endpoint titer was 1:5,120.
Dromedary camels that had MERS-CoV antibodies were present at all sampling sites and during the 20-year sampling period (Table 1; Figure). With the exception of 1 county, seroprevalence was generally higher in the Northeastern and Eastern regions (range 53.4%–100%) than in the northern Rift Valley region (range 0%–17.5%).
Serum samples from 28 dromedary camels from Wajir County that had been held at a research center in isolation conditions since 1998 were negative for MERV-CoV antibodies. To further confirm the observed seropositivity gradient, we compared those 129 camel serum samples with those that were obtained in the same year (2000) but at 2 locations (Eastern and northwestern Rift Valley regions). Antibody levels of nomadic dromedary camels from the Eastern region were significantly higher than those for farmed animals from the Rift Valley (corrected χ2 34.1, p<0.005) (Table 2). Adult animals in both regions had a 7%–10% higher seroprevalence than juvenile animals, which is consistent with results of a previous study (6).
Because virus transmission might be influenced by population density, we attempted to correlate seroprevalence with dromedary camel population density across different regions. Data for dromedary camel density (Technical Appendix) were calculated on the basis of livestock counts conducted by the Department of Resource Surveys and Remote Sensing as part of an ongoing Kenya-wide rangeland monitoring program (14). Increased seroprevalence showed a significant correlation (Spearman rank correlation coefficient 0.715, p<0.005) with higher densities of dromedary camel populations in the Northeastern region and the northern part of the Eastern region (range 0.73–2.9 animals/km2) than in the Rift Valley region (0.58–0.6 animals/km2) (Figure; Technical Appendix).
The present study showed that dromedary camels from Kenya have antibodies against MERS-CoV, which complements the current finding that MERS-CoV is a common pathogen in dromedary camel populations (5,6,8,9,13). Our finding of MERS-CoV antibodies in dromedary camels as early as 1992 is consistent with findings of a recent report from Saudi Arabia, which suggested that MERS-CoV has been circulating in dromedary camels for ≥20 years (5).
To project and potentially control virus spread, the public health community must understand factors determining virus maintenance. Our group and others have demonstrated that young dromedary camels have lower seroprevalences and are more likely to carry infectious virus (5,6). Similar observations have been made for coronaviruses in their original chiropteran hosts wherein strong virus amplification occurred soon after the time of parturition (15). Young, immunologically naive animals may thus facilitate virus amplification in dromedary camel populations.
We also demonstrated that dromedary camel population density shows a positive correlation with MERS-CoV seropositivity, which suggests efficient MERS-CoV maintenance or spread if herd density is high. Different types of animal husbandry in the Northeastern and Eastern regions of Kenya might be better predictors of virus transmission among camels. Dromedary camels in this area are often nomadic following rainfall patterns, and are taken across borders into neighboring countries, such as Ethiopia, for trade purposes (13). The observed increase in seropositivity from the Western region to the Northeastern and Eastern regions could be attributed to increased animal-to-animal contact in cross-border dromedary camel metapopulations.
Conversely, dromedary camels that originated in the Northeastern region but had been held in isolation since 1998 were negative for MERS-CoV antibodies, which is consistent with absence of antibodies in dromedary camels bred in isolation in Dubai (6). The combination of nomadic husbandry for a large population and presence of young virus-susceptible animals might facilitate virus maintenance. However, our retrospective study with archived samples could not assess hypotheses for each of the individual variables to determine their relative and absolute degrees of influence on virus circulation.
Because exportation of dromedary camels is largely unidirectional from eastern Africa into the Arabian Peninsula (11), our findings might facilitate the search for more ancestral MERS-CoV variants to clarify the natural history of acquisition of MERS-CoV by dromedary camels and its putative transmission to humans. Our recent finding of a MERS-CoV ancestor in bats from South Africa (3) highlights the need for wider investigations of viral reservoirs. The fact that no human MERS cases have been observed in eastern Africa could indicate less transmissibility of viruses in regional lineages or lack of detection and reporting of cases. Serosurveys of persons handling dromedary camels in this region could help to determine whether silent or unrecognized infections are being maintained in humans.
Dr Corman is a physician and virologist at the Institute of Virology in Bonn, Germany. His research interests include characterization of novel human and zoonotic viruses and development of molecular diagnostic assays.
Acknowledgments
We thank Stephan Kallies, Monika Eschbach-Bludau, Sebastian Brünink, Tobias Bleicker, and Andrea Sieberg for providing excellent technical assistance; Chris Field, the late Jasper Evans, Martin Evans, Gilfrid Powys, and all camel owners and herdsmen for providing help during field work in Kenya; the Kenyan Department of Veterinary Services for providing support during field work; and the Director of the Department of Resource Surveys and Remote Sensing for providing data on camel populations in Kenya.
This study was supported by the European Commission (FP7-EMPERIE no. 223498 and FP7-ANTIGONE no. 278976) and the German Research Foundation (DFG grant DR772/3-1 to C.D.) and the Consultative Group for International Agricultural Research Program on Agriculture for Nutrition and Health. A.L. was supported by the Centrum of International Migration. C.D. received infrastructural support from the German Center for Infection Research.
References
- World Health Organization. Middle East respiratory syndrome coronavirus—update. April 20, 2014 [cited 2014 Apr 26]. http://www.who.int/csr/don/2014_04_20_mers/en/
- Reusken CB, Lina PH, Pielaat A, de Vries A, Dam-Deisz C, Adema J, Circulation of group 2 coronaviruses in a bat species common to urban areas in western Europe. Vector Borne Zoonotic Dis. 2010;10:785–91. DOIPubMedGoogle Scholar
- Ithete NL, Stoffberg S, Corman VM, Cottontail VM, Richards LR, Schoeman MC, Close relative of human middle East respiratory syndrome coronavirus in bat, South Africa. Emerg Infect Dis. 2013;19:1697–9. DOIPubMedGoogle Scholar
- Annan A, Baldwin HJ, Corman VM, Klose SM, Owusu M, Nkrumah EE, Human betacoronavirus 2c EMC/2012-related viruses in bats, Ghana and Europe. Emerg Infect Dis. 2013;19:456–9. DOIPubMedGoogle Scholar
- Alagaili AN, Briese T, Mishra N, Kapoor V, Sameroff SC, de Wit E, Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. MBio. 2014;5:e00884–14. DOIPubMedGoogle Scholar
- Meyer B, Muller MA, Corman VM, Reusken CB, Ritz D, Godecke GJ, Antibodies against MERS coronavirus in dromedaries, United Arab Emirates, 2003 and 2013. Emerg Infect Dis. 2014;20:552–9. DOIPubMedGoogle Scholar
- Perera RA, Wang P, Gomaa M, El-Shesheny R, Kandeil A, Bagato O, Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveill. 2013;18:20574 .PubMedGoogle Scholar
- Reusken CB, Haagmans BL, Muller MA, Gutierrez C, Godeke GJ, Meyer B, Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13:859–66. DOIPubMedGoogle Scholar
- Haagmans BL, Al Dhahiry SH, Reusken CB, Raj VS, Galiano M, Myers R, Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14:140–5. DOIPubMedGoogle Scholar
- Chu DK, Poon LM, Gomaa MM, Shehata MM, Perera RA, Abu Zeid D, MERS coronaviruses in dromedary camels, Egypt. Emerg Infect Dis. 2014;20:1050–4. DOIGoogle Scholar
- Aklilu Y, Catley A. Livestock exports from the Horn of Africa: an analysis of benefits by pastoralist wealth group and policy implications. Medford (MA): Feinstein International Center, Tufts University; 2009.
- Mahmoud HA. Camel marketing in the northern Kenya/southern Ethiopia borderlands. FAC Research Update 005 DFID, 2010 [cited 2014 Apr 11]. http://www.future-agricultures.org
- Memish ZA, Cotten M, Meyer B, Watson SJ, Alsahafi AJ, Al Rabeeah AA, Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20:1016–9. DOIGoogle Scholar
- Ottichilo W, Grunblatt J, Said M, Wargute P. Wildlife and livestock population trends in the Kenya rangeland. In: Prins HT, Grootenhuis J, Dolan T, editors. Wildlife conservation by sustainable use. Amsterdam: Springer; 2000. p. 203–18.
- Drexler JF, Corman VM, Wegner T, Tateno AF, Zerbinati RM, Gloza-Rausch F, Amplification of emerging viruses in a bat colony. Emerg Infect Dis. 2011;17:449–56 and . DOIPubMedGoogle Scholar
Figure
Tables
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 20, Number 8—August 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Christian Drosten, Institute of Virology, University of Bonn Medical Centre, Sigmund Freud Strasse 25, 53105 Bonn, Germany
Top