Volume 21, Number 2—February 2015
Dispatch
Vesicular Stomatitis Virus–Based Vaccines against Lassa and Ebola Viruses
Figure
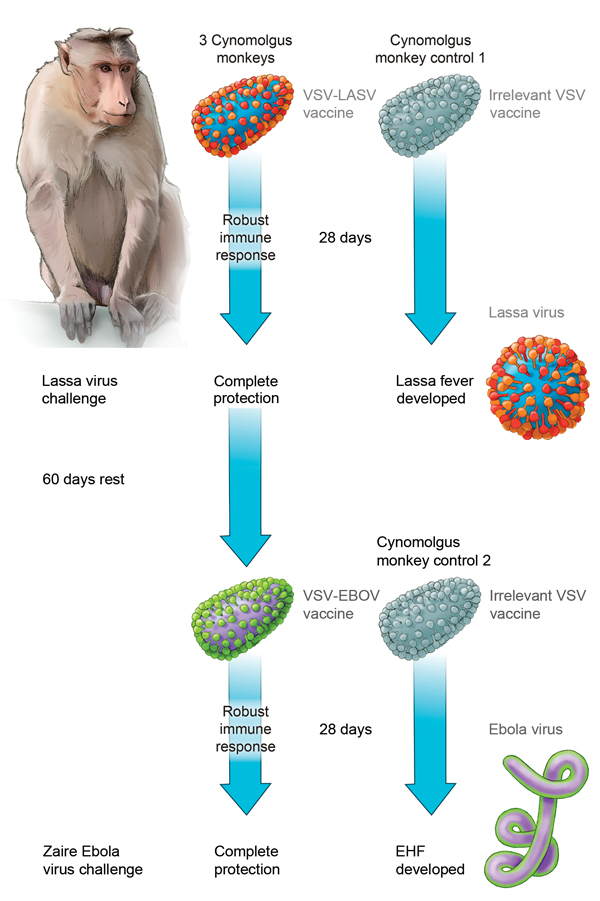
Figure. Effect of sequential vaccination with recombinant vesicular stomatitis virus (VSV)–based vaccines on protective efficacy afforded by each vaccine in nonhuman primates. Vaccination with a VSV-based Lassa virus vaccine encoding the Lassa virus glycoproteins provides complete and possibly sterile immunity against a lethal Lassa virus (LASV) challenge. Approximately 90 days after receiving the initial VSV–Lassa vaccine, animals were vaccinated with a VSV-based Ebola virus (EBOV) vaccine. Although we observed a robust VSV-specific immune response, the VSV–Ebola virus vaccine provided complete and possibly sterile immunity against a lethal Zaire Ebola virus challenge. EHF, Ebola hemorrhagic fever.
Page created: January 21, 2015
Page updated: January 21, 2015
Page reviewed: January 21, 2015
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.