Volume 21, Number 5—May 2015
Dispatch
Canine Infections with Onchocerca lupi Nematodes, United States, 2011–2014
Abstract
Infections with Onchocerca lupi nematodes are diagnosed sporadically in the United States. We report 8 cases of canine onchocercosis in Minnesota, New Mexico, Colorado, and Florida. Identification of 1 cytochrome c oxidase subunit 1 gene haplotype identical to 1 of 5 from Europe suggests recent introduction of this nematode into the United States.
The number of human cases of zoonotic filariasis is increasing across industrialized countries (1). In particular, a major zoonotic potential has been recently recognized for Dirofilaria immitis and D. repens nematodes that infect dogs; both of these nematodes have been reported in cases of human dirofilariasis in the Western Hemisphere and the Old World (1,2).
After the first description of Onchocerca lupi nematodes in 1967 in a Caucasian wolf (Canis lupis cubanensis) from Georgia (former Union of Soviet Socialist Republics), this nematode has been recognized as the causative agent of canine and feline onchocercosis (3,4). In dogs, the infection occurs in an acute or chronic form characterized by ocular nodules that are often evident on the eyelids, conjunctiva, and sclera (3,5). However, if nematodes localize in the retrobulbar space of the eye, the infection may remain undetected (6). Nonetheless, in disease-endemic areas, O. lupi microfilariae may be isolated from skin sediments of apparently healthy dogs (7). Thus, dogs with overt ocular infections might represent only a small portion of the population in which canine onchocercosis occurs in countries such as Hungary, Greece, Germany, and Portugal (3,7).
The role of O. lupi nematodes as an agent of infection in dogs in the United States has been suspected. However, nematodes were previously identified only as Onchocerca sp. in California and Utah (8,9) or as O. lienalis in Arizona (10). Recent etiologic delineation of O. lupi nematodes in dogs and cats in southwestern states (4,11,12) suggested involvement of this parasite in previous cases.
After the first case report of human ocular onchocercosis caused by O. lupi nematodes in Turkey (13), interest in this parasite has been renewed, and additional zoonotic cases have been identified in Turkey, Tunisia, and Iran (14). In addition, this parasite has been extracted from the cervical channel of a 22-month-old child in Arizona (12). Information on the epidemiology and life history of O. lupi nematodes is still minimal, and data on its distribution in the United States is limited to 6 case reports (4,11).
We report 8 cases of O. lupi nematode infection in dogs from Minnesota, New Mexico, Colorado, and Florida. We also compare cytochrome c oxidase subunit 1 (cox1) gene sequences from 2 nematodes with sequences from parasites in Europe to determine possible recent introduction of this filarioid from Europe to the United States.
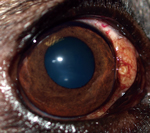
During April 2011–August 2014, a total of 8 privately owned dogs of various ages and sexes were referred to clinical practices in Minnesota (n = 1), New Mexico (n = 4), Colorado (n = 2), and Florida (n = 1) because of different degrees of ocular alterations (Table). At physical examination, nodules were detected in different areas of the eye (Figure 1) and associated with inflammatory reactions ranging from mild scleritis to episcleral swelling and vascular congestion (Table).
All nodules were surgically removed from bulbar conjunctiva or sclera, and white filaria-like parasites were collected and stored in 70% ethanol for morphologic identification. In addition, specimens extracted from 2 dogs (dogs 2 and 3) (Table) were characterized by using molecular techniques. All dogs were treated with macrofilaricides, microfilaricides, antimicrobial drugs, and corticosteroids, which lead to complete resolution of ocular conditions in all except 3 animals (dogs 1, 7, and 8). These 3 dogs had relapses 2, 6, and 12 months, respectively, after surgery.
Nematodes had external, round, transverse ridges and 2 transverse striae per each outer ridge interval, which suggested that they were filarial worms of the genus Onchocerca. The ratio between body diameter and distances between ridges (7–10:1) was specific for O. lupi nematodes (15). A small piece of nematode was used for molecular identification. Genomic DNA was extracted and partial cox1 genes were amplified and sequenced as described (13).
In accordance with clinical signs of nodular ocular lesions and morphologic identification, partial cox1 gene sequence analysis (GenBank accession nos. KP283476 and KP283477) confirmed the identity of the nematode as O. lupi, showing 98% nt homology with other sequences of O. lupi nematodes in GenBank (KC686701 from Portugal and KC686702 from Greece) and 100% with those derived from dogs and cats from the United States, as well as with a sequence from Greece (EF521409).
Phylogenetic analysis of partial cox1 gene sequences was performed by using the neighbor-joining method and the Kimura 2-parameter model in MEGA5 (http://www.megasoftware.net/). This analysis confirmed that sequences from nematodes examined clustered with O. lupi sequences from different areas of the United States (Nevada, California, Colorado, Utah) and with a sequence from Greece (Figure 2). In addition, these sequences were grouped with others from Greece, Hungary and Portugal and formed a paraphyletic clade with other Onchocerca species available in GenBank.
Our results indicate that a unique haplotype of O. lupi nematodes is circulating in the United States and is endemic to the canine population in this country. Although this onchocercid has been implicated as the causative agent of canine onchocercosis in the United States only recently (11), previous cases attributed to Onchocerca spp. have been described in dogs from Arizona, California, and Utah (5,8–10). The cases herein reported from Florida, New Mexico, and Minnesota suggest that the distribution of this nematode is probably wider than previously believed. Detection of O. lupi nematodes in Englewood, Colorado, confirms a previous report of infection in a dog from Mancos (11).
We identified 1 cox1 haplotype and found that it was identical to all sequences in GenBank from the United States and 1 from Greece. Conversely, up to 5 haplotypes were detected in Greece, Turkey, Iran, and Hungary (7). Genetic variation detected in O. lupi nematodes from Europe, Turkey, and Iran, along with isolation of this parasite from the Caucasian wolf, suggests that the infection probably originated in the Old World and was imported into the United States.
The low genetic distance detected for the cox1 gene is evidence of a substantially reduced evolutionary rate, which supports relatively recent divergence among specimens found in the Old World and New World. In addition to recent detection of O. lupi nematode infections in the United States, circulation of 1 haplotype could also suggest that a unique vector species occurs in areas of the Old World and New World where the infection has been diagnosed.
Given that all reports above are based on clinical signs, the epidemiology of O. lupi nematodes in the United States deserves to be thoroughly investigated. In particular, dogs relocated from disease-endemic areas to new areas should be routinely screened for skin-dwelling microfilariae because these parasites might represent a risk for other animals. In addition, because O. lupi nematodes circulate among canine populations, the potential role of dogs as reservoirs for human infection should not be underestimated, as also inferred by zoonotic cases reported in the United States (12). Finally, further studies are urgently warranted toward improving the diagnosis of O. lupi nematode infections, which will lead to a better appreciation of its distribution and potential risk for human populations.
Dr. Otranto is a professor in the Department of Veterinary Medicine, University of Bari, Valenzano, Italy. His research interests include biology and control of arthropod vector-borne diseases of animals and humans.
References
- Otranto D, Eberhard ML. Zoonotic helminths affecting the human eye. Parasit Vectors. 2011;4:41.PubMedGoogle Scholar
- Bowman DD. Heartworms, macrocyclic lactones, and the specter of resistance to prevention in the United States. Parasit Vectors. 2012;5:138.PubMedGoogle Scholar
- Sréter T, Széll Z. Onchocercosis: a newly recognized disease in dogs. Vet Parasitol. 2008;151:1–13. DOIPubMedGoogle Scholar
- Labelle AL, Daniels JB, Dix M, Labelle P. Onchocerca lupi causing ocular disease in two cats. Vet Ophthalmol. 2011;14:105–10. DOIPubMedGoogle Scholar
- Zarfoss MK, Dubielzig RR, Eberhard ML, Schmidt KS. Canine ocular onchocerciasis in the United States: two new cases and a review of the literature. Vet Ophthalmol. 2005;8:51–7 . DOIPubMedGoogle Scholar
- Franchini D, Giannelli A, Di Paola G, Cortes H, Cardoso L, Lia RP, Image diagnosis of zoonotic onchocercosis by Onchocerca lupi. Vet Parasitol. 2014;203:91–5. DOIPubMedGoogle Scholar
- Otranto D, Dantas-Torres F, Giannelli A, Latrofa MS, Papadopoulos E, Cardoso L, Zoonotic Onchocerca lupi infection in dogs, Greece and Portugal, 2011–2012. Emerg Infect Dis. 2013;19:200–3. DOIPubMedGoogle Scholar
- Orihel TC, Ash LR, Holshuh HJ, Santenelli S. Onchocerciasis in a California dog. Am J Trop Med Hyg. 1991;44:513–7 .PubMedGoogle Scholar
- Gardiner CH, Dick EJ Jr, Meininger AC, Lozano-Alarcón F, Jackson P. Onchocerciasis in two dogs. J Am Vet Med Assoc. 1993;203:828–30 .PubMedGoogle Scholar
- Eberhard ML, Ortega Y, Dial S, Schiller CA, Sears AW, Greiner E. Ocular Onchocerca infections in two dogs in western United States. Vet Parasitol. 2000;90:333–8. DOIPubMedGoogle Scholar
- Labelle AL, Maddox CW, Daniels JB, Lanka S, Eggett TE, Dubielzig RR, Canine ocular onchocercosis in the United States is associated with Onchocerca lupi. Vet Parasitol. 2013;193:297–301. DOIPubMedGoogle Scholar
- Eberhard ML, Ostovar GA, Chundu K, Hobohm D, Feiz-Erfan I, Mathison BA, Zoonotic Onchocerca lupi infection in a 22-month-old child in Arizona: first report in the United States and a review of the literature. Am J Trop Med Hyg. 2013;88:601–5. DOIPubMedGoogle Scholar
- Otranto D, Sakru N, Testini G, Gürlü VP, Yakar K, Lia RP, Case report: first evidence of human zoonotic infection by Onchocerca lupi (Spirurida, Onchocercidae). Am J Trop Med Hyg. 2011;84:55–8. DOIPubMedGoogle Scholar
- Otranto D, Dantas-Torres F, Brianti E, Traversa D, Petrić D, Genchi C, Vector-borne helminths of dogs and humans in Europe. Parasit Vectors. 2013;6:16.
- Mutafchiev Y, Dantas-Torres F, Giannelli A, Abramo F, Papadopoulos E, Cardoso L, Redescription of Onchocerca lupi (Spirurida: Onchocercidae) with histopathological observations. Parasit Vectors. 2013;6:309.
Figures
Table
Cite This ArticleTable of Contents – Volume 21, Number 5—May 2015
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Domenico Otranto, Università degli Studi di Bari, Strada Provinciale per Casamassima km 3, Valenzano 70010, Italy
Top