Volume 21, Number 8—August 2015
Dispatch
Genomic Assays for Identification of Chikungunya Virus in Blood Donors, Puerto Rico, 2014
Abstract
A newly developed transcription-mediated amplification assay was used to detect chikungunya virus infection in 3 of 557 asymptomatic donors (0.54%) from Puerto Rico during the 2014–2015 Caribbean epidemic. Viral detection was confirmed by using PCR, microarray, and next-generation sequencing. Molecular clock analysis dated the emergence of the Puerto Rico strains to early 2013.
Chikungunya virus (CHIKV), a mosquitoborne alphavirus, family Togaviridae, causes an acute illness, manifested as fever and severe arthralgia (1). CHIKV infections are associated with global epidemics, and cases reemerged in the Americas in December 2013 after an ≈200-year absence (1,2). The initial cases were reported from the island of Saint Martin in the Caribbean, with autochthonous cases reported across 9 islands by April 2014. By March 2015, >1,250,000 suspected or confirmed cases had been reported in the Americas (http://www.paho.org/hq/index.php?Itemid=40931), including ≈3,200 cases in North America, most (89%) in returning travelers.
Risk for transfusion-transmitted infection (TTI) of CHIKV is currently unclear. However, several factors raise concern about possible CHIKV TTI, including a 10%–25% asymptomatic infection rate and high viremic titers in asymptomatic persons (3). Recently, a probable TTI case from Ross River virus (RRV), an alphavirus related to CHIKV, was reported in a person who had received RRV-positive donor blood, and a clinically compatible illness developed with subsequent seroconversion (4).
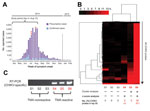
A prototype CHIKV transcription-mediated amplification (TMA) assay was used to screen blood donors from Puerto Rico during the peak of the 2014 Caribbean epidemic (Table). After routine blood donation to the American Red Cross April 4–August 14, 2014, frozen surplus plasma samples from all donors were de-identified and retained for study (all collected during the peak weeks of the 2014 CHIKV outbreak; http://www.salud.gov.pr/Estadisticas-Registros-y-Publicaciones/Pages/Chikungunya.aspx) (Figure 1, panel A). Each retained sample tested negative for pathogens on all required donation screening tests and was also negative for investigational dengue virus (DENV, types 1–4) RNA by TMA (8). Passive reporting was encouraged by use of a donor information sheet describing signs/symptoms of DENV and CHIKV infection. No donor reported any symptoms of arbovirus infection from the time of collection through 12 days following donation. The 557 samples were screened with a candidate screening real-time TMA CHIKV assay with a 95% limit of detection of 16.27 RNA copies/mL (95% CI 11.10–29.56 copies/mL) on the high-throughput automated Panther system (Hologic, Inc., San Diego, CA, USA). Each sample was tested in singlet; reactive samples were diluted 1:16 and logarithmically from 10−2 to 10−8 and retested in triplicate. Three samples (0.54%) were CHIKV RNA–reactive by TMA, with estimated viral loads ranging from 2.9 × 105 to 9.1 × 107 copies/mL (Table). One sample corresponded to a donor who had a confirmed diagnosis of CHIKV infection when contacted after the 12-day reporting period (7.6 × 105 copies/mL); the other 2 donors remained asymptomatic.
For confirmation, we performed blinded orthogonal panviral microarray (ViroChip, University of California San Francisco, San Francisco, CA, USA) and PCR testing of 6 samples, the 3 positive for CHIKV and 3 randomly selected negative controls. (ViroChip is a DNA-detection microarray containing 57,444 probes, and the latest version (v. 5.0) represents all viruses in GenBank as of December 2010 [9]). Nucleic acid extraction was performed from 400 μL of TRIzol-inactivated donor serum by using the Direct-zol RNA MiniPrep Kit (Zymo Research, Irvine, CA, USA), and on-column treatment was performed with Turbo DNase (Life Technologies, Carlsbad, CA, USA). After microarray processing, ViroChip hybridization patterns were analyzed by using hierarchical clustering and z-score analysis (6). Each of the 3 TMA-positive samples was positive for CHIKV by ViroChip by one or both analysis methods (Figure 1, panel B), whereas all 3 controls tested negative by ViroChip. Given the presence of sparse cross-hybridization artifacts in individual microarray probes (Figure 1, panel B), we further tested the samples using a previously reported CHIKV PCR assay (7), which generated results 100% concordant with those of TMA (Figure 1, panel C).
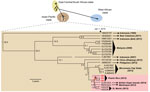
We then used unbiased metagenomic next-generation sequencing (NGS) (9) as a pan-pathogen screen and to recover the viral genome from the 3 CHIKV-positive samples (Figure 1, panel D). NGS libraries were constructed by using the Nextera XT kit (Illumina, San Diego, CA, USA) and validated as described (10), followed by 161-bp, single-end sequencing on an Illumina MiSeq instrument. Raw NGS data (3.2–32.4 million reads per sample) were analyzed for reads corresponding to known pathogens by using the sequence-based ultrarapid pathogen identification (SURPI) computational pipeline (10). After computational subtraction of human host reads, alignment was performed against all microbial sequences in the National Center for Biotechnology Information nucleotide database and the best hit selected on the basis of percentage of mapped read coverage and pairwise identity. A Caribbean strain of CHIKV from the British Virgin Islands (accession no. KJ451624) (11) was identified by SURPI as the closest matching viral genome; 95%–100% genome coverage was obtained for the 3 CHIKV-positive donors (Figure 1, panel D). Phylogenetic analysis of the 3 Puerto Rico CHIKV genomes, together with all 188 publicly available sequenced CHIKV genomes in the reference database, placed the Puerto Rico strains in the Caribbean clade (Figure 2, panels A, B). Molecular clock analysis revealed that this clade, an offshoot of the Southeast Asian/Pacific lineage, possibly recently emerged in the Western Hemisphere in early 2013, with the 3 Puerto Rico viruses diverging from the other Caribbean strains 1.7 years ago (Figure 2, panel C).
We employed several orthogonal genomic-based assays to detect CHIKV infection by real-time TMA testing in 3 asymptomatic donors during the peak of the 2014–2015 Caribbean epidemic (1,2). We confirmed this finding using specific PCR, microarray, and NGS analyses (9) and tracked the emergence of CHIKV in the Western Hemisphere to early 2013 by NGS-based whole-genome sequencing and molecular clock analysis. The rate of CHIKV positivity in donors from Puerto Rico (3/557, 0.54%) is slightly higher than that previously reported in donors from the French West Indies (4/2,149, 0.2%; p = 0.16 by Fisher 2-tailed exact test) (14). In that study, 2 of 4 CHIKV-positive donors had febrile illness 12–24 hours postdonation, whereas fever and joint pains developed in 1 of 3 CHIKV-positive donors in our study. The level of viral RNA in the Puerto Rico donor from with the highest titer, 9.1 × 107 copies/mL, who remained asymptomatic, is comparable to the median viral titer observed previously in symptomatic CHIKV patients (5.6 × 105 PFU/mL, or ≈5.6 × 107 copies/mL) (3). No cases of CHIKV-associated TTI have been confirmed to date, although potential transmission by that route of related alphaviruses such as RRV has been documented (4). Nevertheless, our results indicate that high-titer asymptomatic CHIKV infections, if transmissible by transfusion, can readily elude routine screening based solely on postdonation reporting of febrile illness.
New genomic-based technologies have utility for outbreak investigation, bloodborne pathogen screening, and disease surveillance (9). The availability of a high-throughput TMA assay will facilitate screening for CHIKV and more precisely establish the risk of transfusion-associated transmission. Panviral microarrays are useful for broad surveillance of bloodborne pathogens (9), yet rigorous individual probe validation across multiple targets is needed because of potential cross-hybridization artifacts. Metagenomic NGS (9,10) is an unbiased diagnostic method that identifies all potential pathogens simultaneously on the basis of uniquely identifying DNA sequences. In our study, metagenomic NGS and SURPI analysis facilitated rapid identification and whole-genome recovery of 3 Puerto Rican CHIKV strains directly from primary samples without the need for viral culture.
Recovery from CHIKV infection appears to confer lifelong immunity, and thus an unknown but potentially large fraction of the population of the Puerto Rico may be immune. However, the ongoing threat to returning travelers and spread of the mosquito vector to immunologically naive populations (e.g., in United States and Mexico) underscore the need for continual donor surveillance (15). Increased use of microarrays and NGS in the future would be anticipated, given its suitability for detecting threats from multiple emerging vector-borne diseases such as chikungunya and dengue (2).
Acknowledgments
V. Bres and J.M. Linnen are employees of Hologic Inc. C.Y.C. is the director of the UCSF-Abbott Viral Diagnostics and Discovery Center and receives research support from Abbott Laboratories, Inc. This study was partially supported by grants from the National Institutes of Health (NIH) R01-HL105704 (to C.Y.C.) and Abbott Viral Discovery Award (to C.Y.C.).
Dr. Chiu is an associate professor in laboratory medicine and medicine/infectious diseases at University of California, San Francisco. His interests focus on the development and validation of genomic technologies (microarrays and next-generation sequencing) for clinical diagnosis, blood screening, and outbreak surveillance.
References
- Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383:514. DOIPubMedGoogle Scholar
- Halstead SB. Reappearance of chikungunya, formerly called dengue, in the Americas. Emerg Infect Dis. 2015;21:557–61.PubMedGoogle Scholar
- Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K. Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion. 2013;53:2567–74. DOIPubMedGoogle Scholar
- Hoad VC, Speers DJ, Keller AJ, Dowse GK, Seed CR, Lindsay MD, First reported case of transfusion-transmitted Ross River virus infection. Med J Aust. 2015;202:267–9. DOIPubMedGoogle Scholar
- Greninger AL, Chen EC, Sittler T, Scheinerman A, Roubinian N, Yu G, A metagenomic analysis of pandemic influenza A (2009 H1N1) infection in patients from North America. PLoS ONE. 2010;5:e13381.PubMedGoogle Scholar
- Chiu CY, Rouskin S, Koshy A, Urisman A, Fischer K, Yagi S, Microarray detection of human parainfluenzavirus 4 infection associated with respiratory failure in an immunocompetent adult. Clin Infect Dis. 2006;43:e71–6. DOIPubMedGoogle Scholar
- Pfeffer M, Linssen B, Parke MD, Kinney RM. Specific detection of chikungunya virus using a RT-PCR/nested PCR combination. J Vet Med B Infect Dis Vet Public Health. 2002;49:49–54. DOIPubMedGoogle Scholar
- Stramer SL, Linnen JM, Carrick JM, Foster GA, Krysztof DE, Zou S, Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion. 2012;52:1657–66. DOIPubMedGoogle Scholar
- Stramer SL, Dodd RY, Chiu CY. Advances in testing technology to ensure transfusion safety–NAT and beyond. [ISBT Science Series]. Vox Sang. 2015;10(Suppl 1):55–64. DOIGoogle Scholar
- Naccache SN, Federman S, Veeraraghavan N, Zaharia M, Lee D, Samayoa E, A cloud-compatible bioinformatics pipeline for ultrarapid pathogen identification from next-generation sequencing of clinical samples. Genome Res. 2014;24:1180–92. DOIPubMedGoogle Scholar
- Lanciotti RS, Valadere AM. Transcontinental movement of Asian genotype chikungunya virus. Emerg Infect Dis. 2014;20:1400–2. DOIPubMedGoogle Scholar
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9. DOIPubMedGoogle Scholar
- Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29:1969–73. DOIPubMedGoogle Scholar
- Gallian P, de Lamballerie X, Salez N, Piorkowski G, Richard P, Paturel L, Prospective detection of chikungunya virus in blood donors, Caribbean 2014. Blood. 2014;123:3679–81. DOIPubMedGoogle Scholar
- Farrugia A, Kreil TR. Reflections on the emergence of chikungunya virus in the United States: time to revisit a successful paradigm for the safety of blood-derived therapies. Transfusion. 2015;55:224–6. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 21, Number 8—August 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Susan L. Stramer, American Red Cross, 9315 Gaither Rd, Gaithersburg, MD 20877, USA
Top