Volume 22, Number 3—March 2016
Letter
Generalized Cowpox Virus Infection in a Patient with HIV, Germany, 2012
To the Editor: In October 2012, a 35-year-old man with clinical category C HIV infection was admitted to the intensive care unit at the University of Duisburg–Essen, Essen, Germany. The man had severe respiratory distress syndrome with septic shock, and he was infected with hepatitis B and C viruses and Epstein-Barr virus. Standard infection-control procedures were followed: the patient was placed in a single room; healthcare providers wore personal protective equipment (gown, face shield, mask, and gloves); and a closed system was used for endotracheal suctioning.
Physical examination of the patient revealed multiple skin lesions on his right forearm and right leg. In the following days, more skin lesions appeared on his abdomen and head. The skin lesions were inflamed macules with central livid, hemorrhagic ulceration (1–2 cm in diameter) and raised edges. Kaposi sarcoma was suspected initially, but on hospital day 5, a skin biopsy showed large intracellular eosinophilic inclusion bodies pathognomonic for infection with cowpox virus (family Poxviridae, genus Orthopoxvirus). To confirm the diagnosis of cowpox virus infection, we conducted biopsies of 3 skin lesions on hospital day 7. Despite antimicrobial drug and supportive therapy, the patient died that day from septic shock.
The 3 biopsy samples obtained on hospital day 7 were cultured on African green monkey kidney (MA104) cells, and within 2 days, many plaques were observed. DNA extracted from homogenates and virus isolated from the biopsy material were tested by orthopoxvirus real-time PCR (1); results were positive for all 6 samples. We confirmed the presence of cowpox virus DNA in all samples by sequencing the hemagglutinin gene.
Serum obtained from the patient on day 2 after admission, when the first lesions were noted, was also positive for orthopoxvirus DNA by real-time PCR (1); approximately 50 genome copies were detected, corresponding to a cycle threshold of 29.7. No orthopoxvirus-specific IgG was detected by immunofluorescence assay; this lack of detection is in agreement with observations that orthopoxvirus antibodies can first be detected in the pustular stage of disease but not as early as the macular stage (http://www.bt.cdc.gov/agent/smallpox/smallpox-biological-weapon-abstract.asp). The patient was born after the cessation of mandatory smallpox vaccination, so vaccine-induced IgG is unlikely.
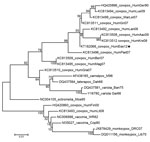
Generalized cowpox virus infection in humans is atypical; the disease usually manifests as a single painful, ulcerated vesiculopustular lesion, which subsequently forms a scar, accompanied by malaise, fever, and long-lasting, painful local lymphadenopathy. However, in immunocompromised persons and persons with eczema, a generalized (and lethal, in at least 1 case) smallpox-like infection can develop (2–7). Phylogenetic analysis, based on the hemagglutinin gene, of the cowpox isolate from this study (GenBank accession no. KT182068) (Figure) revealed a close relationship with cowpox viruses isolated from humans in Germany during 1990 and 2009 (8). This group of viruses forms a distinct clade that is closer to taterapox, camelpox, and variola (smallpox) viruses than to another clade that contains other strains of cowpox virus and vaccinia and monkeypox viruses. Previous reports have postulated the existence of at least 2 species of cowpox virus (8).
Cattle were initially incorrectly presumed to be cowpox virus reservoirs; today, wild rodents are considered to be the true reservoirs. Cowpox virus is transmitted to humans by direct contact with infected animals, mainly cats, which become infected when hunting small rodents. The incubation period is typically 7–12 days. The source of infection for the patient in our study remains unclear; interviews with his family revealed no previous contact with pet animals.
Vaccinia virus, another orthopoxvirus, is known to have induced a generalized infection in a 19-year-old military recruit after smallpox vaccination; the recruit had HIV infection, but this was not known before vaccination (9). Satellite ulcers at the site of inoculation and a widespread, disseminated pustular rash resulted in disseminated vaccinia and AIDS-associated complications that culminated in death of the recruit 18 months after vaccination.
In patients without underlying disease, cowpox infections manifest as self-healing diseases. However, in the absence of vaccination and among a population with increased numbers of immunocompromised persons, the risk for human poxvirus infections is increasing. Early diagnosis is essential for differentiating cowpox from illnesses and skin reactions with similar signs and symptoms, such as smallpox, monkeypox, generalized vaccinia virus infection, disseminated herpes zoster and herpes simplex virus infections, drug-associated eruptions, erythema multiforme, enterovirus infections, secondary syphilis, scabies, insect bites, impetigo, and molluscum contagiosum. The oral drug tecovirimat (previously known as ST-246), as well as cidofovir, CMX–001 (an antiviral substance), and vaccinia immune globulin, should be considered for use as postexposure therapeutic treatment for orthopoxvirus disease (10).
References
- Olson VA, Laue T, Laker MT, Babkin IV, Drosten C, Shchelkunov SN, Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J Clin Microbiol. 2004;42:1940–6 . DOIPubMedGoogle Scholar
- Lawn R. Risk of cowpox to small animal practitioners. Vet Rec. 2010;166:631. DOIPubMedGoogle Scholar
- Pelkonen PM, Tarvainen K, Hynninen A, Kallio ER, Henttonen K, Palva A, Cowpox with severe generalized eruption, Finland. Emerg Infect Dis. 2003;9:1458–61. DOIPubMedGoogle Scholar
- Czerny CP, Eis-Hübinger AM, Mayr A, Schneweis KE, Pfeiff B. Animal poxviruses transmitted from cat to man: current event with lethal end. Zentralbl Veterinarmed B. 1991;38:421–31 .PubMedGoogle Scholar
- Blackford S, Roberts DL, Thomas PD. Cowpox infection causing a generalized eruption in a patient with atopic dermatitis. Br J Dermatol. 1993;129:628–9. DOIPubMedGoogle Scholar
- Hönlinger B, Huemer HP, Romani N, Czerny CP, Eisendle K, Höpfl R. Generalized cowpox infection probably transmitted from a rat. Br J Dermatol. 2005;153:451–3. DOIPubMedGoogle Scholar
- Haase O, Moser A, Rose C, Kurth A, Zillikens D, Schmidt E. Generalized cowpox infection in a patient with Darier disease. Br J Dermatol. 2011;164:1116–8. DOIPubMedGoogle Scholar
- Dabrowski PW, Radonic A, Kurth A, Nitsche A. Genome-wide comparison of cowpox viruses reveals a new clade related to variola virus. PLoS ONE. 2013;8:e79953. DOIPubMedGoogle Scholar
- Redfield RR, Wright DC, James WD, Jones TS, Brown C, Burke DS. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–6. DOIPubMedGoogle Scholar
- Duraffour S, Andrei G, Snoeck R. Tecovirimat, a p37 envelope protein inhibitor for the treatment of smallpox infection. IDrugs. 2010;13:181–91 .PubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 22, Number 3—March 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hermann Meyer, Neuherbergstrasse 11, 80937 Munich, Germany
Top