Volume 22, Number 4—April 2016
Dispatch
Arenavirus Diversity and Phylogeography of Mastomys natalensis Rodents, Nigeria
Abstract
Mastomys natalensis rodents are natural hosts for Lassa virus (LASV). Detection of LASV in 2 mitochondrial phylogroups of the rodent near the Niger and Benue Rivers in Nigeria underlines the potential for LASV emergence in fresh phylogroups of this rodent. A Mobala-like sequence was also detected in eastern Nigeria.
Lassa fever, a viral hemorrhagic disease, is estimated to infect 150,000–300,000 persons every year, killing ≈5,000 (1). Within West Africa, Lassa fever is endemic to 2 regions: 1) Guinea, Sierra Leone, and Liberia; and 2) Nigeria. Even within most of these countries, Lassa fever is endemic to certain areas but rare or completely absent in others (2). Zoonotic disease nidality describes the phenomenon in which geographic occurrence of a zoonotic disease is markedly focused or fragmented, as opposed to occurring continuously or spreading in a consistent pattern (3). Zoonotic disease nidality might result when only select phyletic groups in a host species are capable of serving as reservoirs for the pathogen (4).
The natural host for Lassa virus (LASV), the arenavirus that causes Lassa fever, is the multimammate rat Mastomys natalensis (5). This rodent, which is distributed all over sub-Saharan African, is also host to other arenaviruses such as the Mopeia virus in southeastern Africa (6), Morogoro and Gairo viruses in Tanzania (7,8), and Luna virus in Zambia (9).
In a genetic study of M. natalensis rodents across Africa (10), which analyzed cytochrome b sequences, researchers found that populations of these rodents in western Africa belong to the same monophylogenetic phylogroup, A-I. However, those authors detected phylogroup A-I of M. natalensis rodents in countries west of Nigeria and phylogroup A-II in countries east of Nigeria, but they did not sample Nigeria, the contact zone for rodents of these phylogroups. As a country in which Lassa fever is endemic in the western and eastern areas (2), Nigeria presents an excellent opportunity for investigation of patterns of LASV and arenavirus occurrence in 2 phylogroups of M. natalensis rodents. Our objectives in this study were to 1) determine which M. natalensis rodent cytochrome b phylogroups (A-I and A-II) are infected with LASV and other arenaviruses, and 2) identify the limits of distribution of these phylogroups within Nigeria.
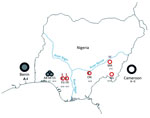
From January 2011 through March 2013, small mammals were captured in H.B. Sherman live animal traps (https://www.shermantraps.com/) at 8 sites across Lassa fever–endemic and –nonendemic areas in Nigeria (Figure 1). We classified Lassa fever–nonendemic areas as areas where no cases of Lassa fever have been documented (2). Permission to trap rodents in various localities was granted by the Ministry of Environment, Osun State; Gwer West Local Government Council, Benue State; and the Ministry of Health, Taraba State.
Among 782 small mammals, 274 M. natalensis rodents were trapped. Identification of the animals in the field was based on external morphology and later confirmed genetically by cytochrome b gene sequencing. The rodents were euthanized, and biopsy samples (blood, liver, kidneys, spleen) were collected for laboratory analyses. Precautions for working with animals potentially infected with dangerous pathogens were strictly followed (11).
Using a QIAamp Viral RNA Mini Kit (QIAGEN, Valencia, CA, USA), we extracted total RNA from 20 μL of whole blood frozen at −80°C. Extracted RNA was tested with a panarenavirus protocol designed to amplify the L (polymerase) gene (340 nt) (12) and with another reverse transcription PCR specific for LASV, selective for the glycoprotein precursor (GPC) gene (303 nt) (13). We conducted further PCR amplification of the GPC fragment (using primers in Technical Appendix 1 Table) for specimens positive on initial screening. Phylogenies were inferred by use of the Bayesian Markov Chain Monte Carlo method implemented in BEAST version 1.6.2 (http://beast.bio.ed.ac.uk/).
Of the 274 M. natalensis rodents from the 8 sampled sites, 16 were positive by PCR for arenavirus (Figure 1; Table). Phylogenetic analyses of the GPC and L gene sequences showed that 15 of the viruses were Lassa and 1 was a Mobala-like virus (Figure 2). The LASV sequences from Ekpoma and Eguare-Egoro belonged to lineage II and clustered with strains Nig08-A4, A37, A41, and A47 from patients in Edo State (14). Nucleotide identities between the sequences of LASV from the rodents and those from the human patients were 82%–96% (GPC) and 85%–97% (L), and amino acid identities were 95%–99% (GPC) and 93%–100% (L), respectively (Technical Appendix 2). The sequence from Mayo Ranewo was conversely more distant from those from Mobala and Gairo; nucleotides identities were 69%–73% (GPC) and 77%–80% (L), and amino acid identities were 75%–77% (GPC) and 90%–95% (L), respectively (Technical Appendix 2).
Sequence analysis of the region coding cytochrome b indicated that M. natalensis rodents from Nigeria cluster in 2 clades. The first clade corresponds to phylogroup A-I, which clusters with sequences from rodents from Benin, which is west of Nigeria (Figures 1, 2; Technical Appendix 2). Phylogroup A-I, including sequences from Abagboro, Esira, Kako, Eguare-Egoro, Ekpoma from western Nigeria, extends across the Niger and Benue Rivers into Onmba-Abena in eastern Nigeria.
The second clade corresponds to phylogroup A-II, which clustered with sequences from Cameroon, which is east of Nigeria (Figures 1, 2; Technical Appendix 1 Figure). Phylogroup A-II within Nigeria is represented by M. natalensis rodents from Ngel-Nyaki, Mayo-Ranewo, and Onmba-Abena in eastern Nigeria, but this phylogroup also overlaps the Niger and Benue Rivers westward into Eguare-Egoro and Ekpoma. The contact zone between rodents of phylogroups A-I and A-II in Nigeria was detected at sites relatively close to the Niger and Benue Rivers (Eguare-Egoro, Ekpoma, Onmba-Abena) (Figure 1). The Niger River has been demonstrated to be a natural barrier for some rodents (15) but seems to delimit these 2 phylogroups only to an extent. Human-assisted long-distance migration of commensal rodents could influence their genetic structure, which may be what happened for rodents of the same M. natalensis phylogroup that were detected on opposite banks of the Niger River.
M. natalensis phylogroup A-I rodents were infected with LASV in Eguare-Egoro and Ekpoma but not in Abagboro, Kako, and Esira. Because all rodents from these sites belong to the same phylogroup, some factor other than cytochrome b genetic structure might be responsible for the focal prevalence of LASV. It could be, however, that our study was limited by use of the cytochrome b mitochondrial marker only, which is maternally inherited. Therefore, other biparentally inherited genetic markers, such as microsatellites, should be investigated. Environmental variables such as humidity and temperature could also be considered (2).
M. natalensis phylogroup A-II rodents were infected by LASV and a Mobala-like virus. We did not detect any LASV-positive, phylogroup A-II rodents east of the Niger River, although all the sites sampled in this area lie within the Lassa fever–endemic zone and regularly experience epidemics (2). It is worth exploring the possibility that other small mammals might also host LASV. LASV-positive members of phylogroup A-II, however, were found on the west bank of the Niger River in Eguare-Egoro and Ekpoma (along with LASV-positive members of phylogroup A-I). A crucial implication of these findings is the potential that new, previously naive populations and phylogroups of M. natalensis rodents could become infected with LASV and the disease could emerge in new regions in western Africa.
Detection of the Mobala-like virus in M. natalensis rodents within Mayo-Ranewo in eastern Nigeria deserves further study. We included Mayo-Ranewo among our survey sites because an epidemic of hemorrhagic fever, considered but not confirmed to be Lassa fever, occurred there in 2012. Whether the Mobala-like arenavirus detected in this village has pathogenic properties remains to be determined.
Dr. Olayemi is a research fellow in mammalogy at the Natural History Museum, Obafemi Awolowo University, Nigeria, and a postdoctoral fellow of the European Foundation Initiative for African Research into Neglected Tropical Diseases. His research interests include rodent systematics and ecology and how this knowledge can be used to control zoonoses like Lassa fever.
Acknowledgments
We thank Akinola Awoyelu, Destiny Aigbomian, Gideon Igbana, Mustapha, Bala, Sambo Bapetel, Timothy Gyuse, and Hazel Chapman for greatly assisting with our field work.
This research was funded under the project Ecology of Transmission of the Deadly Lassa Virus from Rodents to Humans across Various Flashpoints within Nigeria, via a junior postdoctoral fellowship to A. Olayemi by the European Foundation Initiative for African Research into Neglected Tropical Diseases
References
- McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. DOIPubMedGoogle Scholar
- Fichet-Calvet E, Rogers DJ. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3:e388 . DOIPubMedGoogle Scholar
- Pavloskii EN. Natural nidality of transmissible diseases, with special reference to the landscape epidemiology of zooanthroponoses. Urbana (IL): University of Illinois Press; 1966.
- Salazar-Bravo J, Dragoo JW, Bowen MD, Peters CJ, Ksiazek TG, Yates TL. Natural nidality in Bolivian hemorrhagic fever and the systematics of the reservoir species. Infect Genet Evol. 2002;1:191–9. DOIPubMedGoogle Scholar
- Monath TP, Newhouse VF, Kemp GE. Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science. 1974;185:263–5 Setzer HW and Cacciapuoti A. DOIPubMedGoogle Scholar
- Wulff H, McIntosh BM, Hamner DB, Johnson KM. Isolation of an arenavirus closely related to Lassa virus from Mastomys natalensis in south-east Africa. Bull World Health Organ. 1977;55:441–4 .PubMedGoogle Scholar
- Günther S, Hoofd G, Charrel R, Röser C, Becker-Ziaja B, Lloyd G, Mopeia virus–related arenavirus in natal multimammate mice, Morogoro, Tanzania. Emerg Infect Dis. 2009;15:2008–12. DOIPubMedGoogle Scholar
- Gryseels S, Rieger T, Oestereich L, Cuypers B, Borremans B, Makundi R, Gairo virus, a novel arenavirus of the widespread Mastomys natalensis: genetically divergent, but ecologically similar to Lassa and Morogoro viruses. Virology. 2015;476:249–56. DOIPubMedGoogle Scholar
- Ishii A, Thomas Y, Moonga L, Nakamura I, Ohnuma A, Hangombe B, Novel arenavirus, Zambia. Emerg Infect Dis. 2011;17:1921–4. DOIPubMedGoogle Scholar
- Colangelo P, Verheyen E, Leirs H, Tatard C, Denys C, Dobigny G, A mitochondrial phylogeographic scenario for the most widespread African rodent, Mastomys natalensis. Biol J Linn Soc Lond. 2013;108:901–16. DOIGoogle Scholar
- Mills JN, Yates TL, Childs J, Parmenter RR, Ksiazek TG, Rollin PE, Guidelines for working with rodents potentially infected with hantavirus. J Mammal. 1995;76:716–22. DOIGoogle Scholar
- Vieth S, Drosten C, Lenz O, Vincent M, Omilabu S, Hass M, RT-PCR assay for detection of Lassa virus and related Old World arenaviruses targeting the L gene. Trans R Soc Trop Med Hyg. 2007;101:1253–64. DOIPubMedGoogle Scholar
- Olschläger S, Lelke M, Emmerich P, Panning M, Drosten C, Hass M, Improved detection of Lassa virus by reverse transcription-PCR targeting the 5′ region of S RNA. J Clin Microbiol. 2010;48:2009–13. DOIPubMedGoogle Scholar
- Ehichioya DU, Hass M, Becker-Ziaja B, Ehimuan J, Asogun DA, Fichet-Calvet E, Current molecular epidemiology of Lassa virus in Nigeria. J Clin Microbiol. 2011;49:1157–61. DOIPubMedGoogle Scholar
- Olayemi A, Nicolas V, Gaubert P, Leirs H, Verheyen E. Small mammals, morphology and molecules: tale-bearing tenants of the Nigerian southwestern forest block. In: Daniels J, editor. Advances in environmental research, vol. 8. New York: Nova Science Publishers; 2011. p. 247–67.
Figures
Table
Cite This ArticleTable of Contents – Volume 22, Number 4—April 2016
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ayodeji Olayemi, Natural History Museum, Obafemi Awolowo University, Ile-Ife, Osun State HO220005, Nigeria; ,
Top