Volume 5, Number 3—June 1999
Perspective
Bacterial Vaccines and Serotype Replacement: Lessons from Haemophilus influenzae and Prospects for Streptococcus pneumoniae
Abstract
Conjugate vaccines have reduced the incidence of invasive disease caused by Haemophilus influenzae, type b (Hib), in industrialized countries and may be highly effective against Streptococcus pneumoniae. However, the serotype specificity of these vaccines has led to concern that their use may increase carriage of and disease from serotypes not included in the vaccine. Replacement has not occurred with the use of Hib vaccines but has occurred in trials of pneumococcal vaccines. Mathematical models can be used to elucidate these contrasting outcomes, predict the conditions under which serotype replacement is likely, interpret the results of conjugate vaccine trials, design trials that will better detect serotype replacement (if it occurs), and suggest factors to consider in choosing the serotype composition of vaccines.
Conjugate vaccines are a major advance in the control of diseases caused by two members of the normal bacterial flora of the human nasopharynx, Streptococcus pneumoniae (pneumococcus) and Haemophilus influenzae type b (Hib). In the absence of widespread vaccination, pneumococci have been responsible for an estimated 7 million cases of otitis media, 500,000 cases of pneumonia, 50,000 cases of bacteremia, and 3,000 cases of meningitis each year in the United States (1). Before the widespread use of conjugate vaccines, Hib caused invasive disease in an estimated 1 in 200 children <5 years of age in the United States (2). Conjugate vaccines have reduced the incidence of invasive Hib disease by 90% or more in industrialized countries (2,3). After promising phase-II clinical trials (4-7), the first results from a phase-III trial of a pneumococcal conjugate vaccine have shown very high efficacy against invasive disease (8).
In addition to protecting against disease, conjugate vaccines protect against asymptomatic carriage of the target organisms (4-7,9). H. influenzae and S. pneumoniae are frequently found in the normal nasopharyngeal flora of healthy persons, with invasive disease being relatively rare compared with asymptomatic carriage (10,11). Asymptomatic carriers are also responsible for most transmission of these organisms (10,11); in contrast to many other vaccine-preventable infections, disease caused by these organisms seems to contribute little to the process of transmission (12-14). By reducing the rate of carriage of targeted bacteria, conjugate vaccines also reduce their transmission and should thereby offer protection to unvaccinated contacts of vaccinated persons. It has been shown (in the case of Hib) and suggested (in the case of pneumococcus) that the use of conjugate vaccines results in herd immunity (15). Herd immunity may explain why the reduction in invasive Hib disease in some populations has exceeded the fraction of the population that received the vaccine (3) and why Hib invasive disease declined even in age groups that had not yet received the vaccine (16).
Although the reduction in carriage achieved by conjugate vaccines is beneficial from the perspective of herd immunity, it has raised concerns about the possibility of serotype replacement. Both H. influenzae and pneumococci are characterized by extensive antigenic diversity in their polysaccharide capsules. In H. influenzae, six capsular types are known, in addition to a large group of nontypeable (unencapsulated) variants. Before vaccination, serotype b was responsible for most invasive diseases, with minor contributions from the other encapsulated types. Because of the importance of Hib in invasive disease, vaccination efforts have concentrated on the b serotype (16). Pneumococci are even more diverse, with 90 recognized serotypes; many of these serotypes are capable of causing invasive disease. To accommodate this greater diversity, pneumococcal conjugate vaccines have incorporated multiple serotypes. Because the protection offered by conjugate vaccines is specific to the capsular type(s) included in the vaccine, it has been suggested that reducing carriage of these vaccine types may leave open an ecologic niche that will be filled by serotypes not included in the vaccine (5,17-20).
Hib conjugate vaccines served as a model for the development and testing of pneumococcal vaccines. However, pneumococci are epidemiologically different from Hib, and results of clinical trials with pneumococcal conjugates suggest that the two bacteria differ in their response to vaccination, especially with respect to serotype replacement. This article describes how mathematical models can be used to elucidate these contrasting outcomes, specify the conditions under which serotype replacement is likely, interpret the results of conjugate vaccine trials, design trials that will be better able to detect serotype replacement (if it occurs), and suggest factors to consider in choosing the serotype composition of vaccines.
Serotype replacement has not been detected since the introduction of Hib conjugate vaccines. Studies of H. influenzae carriage in 700 children in Finland (21) and 364 families in the United Kingdom (15,22) found no evidence of increased carriage of non-b H. influenzae as a result of vaccination. Although increases in invasive disease from other nasopharyngeal bacteria have been reported since Hib vaccination began (23,24), no evidence of a causal link to Hib vaccination has been observed (22,23). Furthermore, a recent study in the United States showed that the net impact of Hib vaccination has been a 68% reduction in invasive disease from all H. influenzae between 1986 and 1995 (25); therefore, any increase in disease from non-b serotypes is small compared with the reduction in disease from type b.
In contrast, pneumococcal conjugate vaccine studies show considerable evidence of serotype replacement, as measured by nasopharyngeal carriage of nonvaccine type organisms. Increases in the carriage of nonvaccine serotypes have occurred in three major ongoing clinical trials of pneumococcal conjugate vaccines. In Gambia, carriage of nonvaccine serotypes was 79% in children receiving three doses of a pneumococcal conjugate vaccine (compared with 42.5% in controls) (5). In trials of a 9-valent vaccine in South Africa, carriage of nonvaccine serotypes increased from 21% in controls to 39% in vaccine recipients (6). Serotype replacement was observed in the second of two large studies in Israel (4,7); the reason for the difference in outcome between the two studies remains unclear. In the first phase-III trial for which data were presented, no increase was observed in invasive disease from nonvaccine types (8). While this result is encouraging, it may not be indicative of what will occur as conjugate vaccines enter widespread use in a variety of communities.
Mathematical models can be useful in defining the extent of serotype replacement in various contexts, optimizing the design of clinical trials to discern whether such replacement occurs, and interpreting the results of these trials. With these goals in mind, I constructed and analyzed a mathematical model of the transmission dynamics of colonizing bacteria with multiple serotypes, such as pneumococci, and the effect of vaccination on these dynamics. The model is similar in structure to the compartmental models used to design and predict the effects of vaccination programs against other infectious diseases (26).
The main distinguishing feature of this model is that it simultaneously considers the transmission of two (or more) strains of the same organism. The model is designed to analyze the effects of competitive interactions between these strains, in which carriage of one serotype reduces the probability that a host will be colonized with another serotype. If such competitive interactions occur, serotype replacement is possible, because vaccine-induced reductions in some serotypes will increase the opportunities for others to spread in the population. Epidemiologic studies have provided indirect evidence of such competitive interactions (27-29), while laboratory studies have suggested mechanisms by which different species of streptococci (30,31) or different strains of H. influenzae (32) might compete in the nasopharynx. At present, however, little is known about the precise nature of these interactions, and perhaps the most compelling evidence that competition occurs comes from the replacement observed in pneumococcal conjugate vaccine studies.
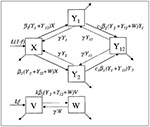
The assumptions and structure of the model are as follows. In the absence of vaccination, the model (Figure 1) assumes that humans are born into the susceptible (X) compartment at a particular rate and are removed from that compartment (and all other compartments) at a specific per capita death (or maturation) rate. Two pneumococcal serotypes (designated 1 and 2) are present, and susceptible hosts may be colonized by either type; colonization moves the host into the Y1 or Y2 compartment, respectively. The incidence of colonization with each type is proportional to the total number of persons carrying that type. Colonization has average duration 1/g. While carrying one serotype, a host may be colonized by the other type, which moves the host into the dually colonized compartment (Y12). This secondary colonization also occurs at a rate proportional to the prevalence of the colonizing type, but a rate that is cj (j = 1 or 2) times the rate at which a susceptible person would be colonized by the same type. Thus, cj is an inverse measure of the competitive inhibition of type j by the resident type in a host.
When vaccination begins, a fraction f of all persons are assumed to be vaccinated at birth. In the model, these persons are born into the vaccinated (V) compartment. It is assumed that vaccination completely protects a person against carriage of type 1 (this is done to simplify the analysis of the model; if only partial protection were offered, the effects would be similar to those observed at a lower level of vaccine coverage f). To consider the effects of including more than one bacterial serotype in the vaccine, the model can accommodate vaccines that are effective only against type 1 (monovalent vaccines), as well as those that give either partial or full protection against type 2 (bivalent vaccines). The parameter k represents the degree of protection offered by the vaccine against serotype 2.
By varying the parameters of the model, it is possible to compare the effects of different levels of vaccine coverage (fractions of the population vaccinated), different assumptions about the competitive interactions among pneumococcal serotypes, and different types of vaccines (monovalent vs. bivalent) (33). In summary, the major predictions of the model are as follows.
If there is competition between different pneumococcal serotypes to colonize hosts, vaccination against serotype 1 alone will increase the prevalence of serotype 2. The extent of replacement, measured as the increase in the prevalence of serotype 2, will be greatest when vaccine coverage is high and when serotype 2 is strongly inhibited from colonizing persons who carry serotype 1. Serotype replacement may take either of two forms: an increase in prevalence of a type already present in the population or the appearance and spread of types previously absent from the population because they were unable to compete with the vaccine type(s).
Bivalent (or polyvalent) vaccines can also cause replacement if the protection offered against different serotypes is uneven. In particular, if a vaccine has relatively low efficacy against serotype 2 but very high efficacy against serotype 1, use of a bivalent vaccine may increase the prevalence of type 2.
If only two serotypes interact in a population, the amount of replacement that can occur is limited. Specifically, the increase in the prevalence of serotype 2 will always be less than or equal to the decrease in the prevalence of serotype 1. Thus, for example, if the prevalences of serotypes 1 and 2 before vaccination are 15% and 20%, respectively, then the prevalence of serotype 2 after vaccination will be no more than 35%.
If more than two types are competing to colonize hosts, this limitation need not hold. In the presence of more than two types, vaccination can increase the prevalence of a single, nonvaccine type more than it reduces the prevalence of the vaccine type.
Although replacement is of concern, it may also be beneficial. If serotypes compete to colonize hosts, increases in the prevalence of the nonvaccine types will help reduce the prevalence of the serotypes included in the vaccine. Thus, replacement will augment the effects of herd immunity in reducing the exposure of all members of the population to vaccine serotypes. This results in a tradeoff between the breadth of coverage of a vaccine (number of serotypes covered) and the effectiveness of the vaccine in reducing carriage of each serotype at the population level.
The model's predictions have several implications for the interpretation of existing data from the use of conjugate vaccines, the design of vaccine trials, and the choice of vaccine composition.
Why Has Replacement Carriage Occurred with Pneumococcal Conjugate Vaccines but Not with Hib Vaccines?
As noted above, the absence of serotype replacement observed with the use of Hib in industrialized countries contrasts with the findings of considerable serotype replacement in two studies of pneumococcal vaccines. What might account for this difference?
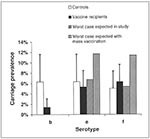
The mathematical model suggests an explanation. The model predicts that, in a pairwise interaction between two serotypes, the increase in prevalence of a nonvaccine type will be no more than the reduction in prevalence of a vaccine serotype. This principle is illustrated in Figure 2, which presents data from a study of Hib conjugate vaccine in the United Kingdom (15). In the figure, the white bars show the prevalence of each of three H. influenzae serotypes—b, e, and f—in vaccinated persons, and the black bars show the prevalence of each of these serotypes in controls. If one assumes that Hib interacts independently with each of the two nonvaccine serotypes (e and f), one can use the two-serotype model to calculate the maximum prevalence of these nonvaccine types in vaccinees that would be expected if these serotypes compete very strongly with serotype b. The striped bars show the maximum prevalence of types e and f expected in the study, where only a small fraction of the community was vaccinated; the shaded bars indicate the equivalent figure if the whole community had been vaccinated. As is clear from the figure, the increase in nonvaccine-type carriage in vaccinees would be minuscule and statistically undetectable in a study of this kind (indeed, the study from which these data were drawn was not designed to detect replacement; data on the prevalence of types e and f were used to control for general changes in the prevalence of H. influenzae that could have been attributable to factors other than vaccination [15]). The reason is that the prevalence of Hib was so low before vaccination that even its complete removal by widespread vaccination would have little effect on competing bacteria. The prevalence of Hib carriage in other industrialized countries is similar to that measured in the UK study. Therefore, the model suggests that the lack of replacement, even after widespread use of the Hib conjugate vaccine in industrialized countries, may be a simple result of the low prevalence of Hib carriage.
If this interpretation is correct, then serotype replacement would be more likely to occur in areas where the prevalence of Hib is higher or for vaccination against other organisms whose prevalence is higher. This difference could account for the contrasting outcomes of vaccination against Hib and pneumococci. Differences in the biology of colonization or in the interactions between bacterial types may also have a role in these contrasting outcomes. Distinguishing the relative importance of these two explanations will require further research into the biologic interactions of bacterial populations in the nasopharynx, as well as studies of the effects of conjugate Hib vaccination in areas where Hib's prevalence is higher.
Detection of Replacement: The Design of Clinical Trials
If used by a large fraction of the human population in a community, a conjugate vaccine may alter the composition of the bacterial population, not only in vaccinated, but also in unvaccinated persons in that community. Vaccination may reduce the prevalence of serotypes included in the vaccine, thereby protecting unvaccinated persons against exposure to these serotypes (herd immunity). Similarly, if serotype replacement occurs and vaccinated persons become more likely to carry nonvaccine serotypes, the exposure of unvaccinated persons to these serotypes will increase. As a result of these indirect effects, strain replacement will be magnified in communities where large numbers of persons are vaccinated.
This process is also evident from Figure 2. There, the striped bars show the model's prediction of the maximum increase in non-vaccine type carriage in vaccine recipients in a community in which the vaccine is used only on a very small proportion of the population, while the shaded bars show the same increase in a community where everyone is vaccinated. As is clear from the figure, replacement will be most easily observed in communities where the level of vaccine coverage is high.
Therefore, one would expect that the extent of serotype replacement when vaccines enter widespread use in a community may be much greater than that observed in clinical trials where a relatively small fraction of the community is immunized. This is one important reason why the failure to observe an increase in invasive disease from nonvaccine-type pneumococci in the Northern California trial (8), while promising, may not be indicative of the potential for replacement once the vaccine is used on a large scale. If one is interested in designing a clinical trial that simulates the selective pressures exerted by communitywide use of a conjugate vaccine, and therefore maximizes the chances of observing serotype replacement during the trial, then community-randomized clinical trials will be superior to individually randomized ones. Studies of pneumococcal vaccines in which communities are the units of randomization are under way in Native American communities in the southwestern United States (K. O'Brien, pers. comm.).
Vaccine Composition: Replacement Revisited
For an organism like pneumococcus, in which a number of serotypes can cause disease, the choice of serotypes for inclusion in a conjugate vaccine is critical. One strategy would be to include as many serotypes as possible to achieve the broadest possible protection. In addition to some clinical limitations on the number of serotypes that can be included in a single vaccine, there are other reasons why such a strategy would not be ideal. As noted above in the last prediction from the model, serotype replacement can augment the effectiveness of a vaccination program in a community. This occurs because increases in the prevalence of nonvaccine serotypes competitively inhibit carriage of vaccine serotypes. Ideally, then, one would like to design a vaccine that maximizes these beneficial effects while minimizing the risk of added disease from increased carriage of nonvaccine serotypes.
The question is how to accomplish such a balance. So far, the model describes only carriage of various serotypes; it does not directly address the problem of disease. The effect of vaccination on disease will depend both on changes in patterns of carriage of different serotypes and on the propensity of the individual serotypes to cause disease. Serotypes of H. influenzae and S. pneumoniae vary considerably in their pathogenicity, as manifested by experimental evidence (34) and by differences between the frequency of particular serotypes in carriage isolates and their frequency in disease isolates (17,35). If these serotype associations were stable, the ideal vaccine could simply include the most pathogenic serotypes but exclude those that tend to be avirulent, thereby taking advantage of any increases in the prevalence of the avirulent serotypes to augment the effect of the vaccine (36).
This approach has several limitations. First, the model predicts that widespread use of a vaccine may result in the appearance of bacterial types which, before vaccination, had been absent from the population because of competition from vaccine types. The virulence of these novel types would be difficult to predict, since competitive inferiority to existing types need not be correlated with low virulence (12,13). Second, both species discussed here are highly transformable. Although capsular type seems to be very closely associated with virulence in H. influenzae (34,37), transformation studies in pneumococci have shown complicated interactions between capsular type and other genes in determining virulence (38), so the existing associations between virulence and capsular type in pneumococci (39,40) may change in response to conjugate vaccine-induced selective pressure. If such vaccines are used on a widespread scale, surveillance of shifts in the serotype associations of invasive disease should be maintained.
Serotype replacement has been discussed primarily as it applies to serotypes not included in the vaccine. However, if the vaccine is only weakly effective in immunizing against carriage of some of the serotypes included in it, even these serotypes may increase in prevalence after vaccination is introduced. This can occur if the efficacy of the vaccine against these serotypes is outweighed by its effect in removing competing serotypes. Results of trials published thus far indicate that the protection offered by the vaccine against included serotypes taken together is considerably lower than 100%. Therefore, the results of future trials should be monitored to determine whether prevalence of any of the individual vaccine serotypes is increasing in vaccinated hosts.
Interpreting Replacement: Is It Real?
Studies of pneumococcal carriage are typically performed by sampling the nasopharyngeal flora of vaccinated and unvaccinated persons, plating the samples on agar, and serotyping one or a few colonies. This technique typically identifies the most abundant pneumococcal serotypes carried by a person, and possibly a minority type if it is present in large numbers. However, many people carry more than one pneumococcal type (27,41), and when the pneumococci are studied in detail, the minority type may be much less plentiful than the majority type—at a frequency of 10% or less (41). Therefore, current methods are likely to have very low sensitivity for the detection of minority types.
This creates a problem in measuring serotype replacement during pneumococcal vaccine trials. Vaccinated persons, who are protected against carriage of vaccine types, may become more susceptible to carriage of pneumococcal types not included in the vaccine. This is serotype replacement, a phenomenon that vaccine trials are intended, in part, to detect. In addition, nonvaccine type pneumococci, even if they are not more plentiful, may be more readily detected in vaccinated persons. Some unvaccinated persons carry both vaccine-type and nonvaccine-type pneumococci, and in some of them, the vaccine-type will be in the majority. Because minority populations of pneumococci are difficult to detect, the nonvaccine-type pneumococci carried by these persons is masked by the vaccine types, resulting in an underestimate of the prevalence of nonvaccine-type pneumococci in the unvaccinated population. Vaccinated persons, by contrast, are less likely to carry vaccine-type pneumococci, so their nonvaccine-type pneumococci are more likely to be detected. This is known as unmasking. Figure 3 illustrates the distinction between serotype replacement and unmasking. Unmasking is an artifact of sampling, and one would like to be able to determine whether a finding of higher nonvaccine-type carriage rates in vaccinated persons reflects true serotype replacement, unmasking, or a combination of these phenomena.
I have recently developed a statistical procedure to answer this question (M. Lipsitch, submitted for publication). The procedure attempts to detect serotype replacement by attempting to reject a null model that incorporates the effect of unmasking alone. In short, if the increase in nonvaccine type carriage in vaccinees, compared to controls, is greater than can be accounted for by this null model, then one concludes that additional factors, presumably serotype replacement, must be responsible for the observed increase. This technique has been applied to two datasets, one from South Africa (6) and one from Gambia (5). In both cases, the observed increase was greater than that expected from unmasking alone. In the South African case, the difference was statistically significant (p = 0.02), but it was not in the Gambian dataset (p = 0.085). However, the Gambian dataset was extremely small and some information was unavailable for this dataset that might have improved the power of the test. The test is simple to perform using the BUGS software (42,43) available free on the World Wide Web (http://www.mrc-bsu.cam.ac.uk/bugs/Welcome.html) and a program available from the author; thus, it may be readily applied to future datasets.
Limitations of Mathematical Models
The mathematical models described here, like all such models, involve a number of simplifications. In some cases, these simplifications are introduced to make the model more tractable and focus attention on fundamental processes of transmission and competition between serotypes. In other cases, the simplifications are necessary because much remains unknown about the biology—and especially the immunology—of carriage of these organisms. The assumptions of the model are discussed at greater length (33). One of these assumptions will be considered here in greater detail to highlight some areas where additional knowledge of the biology of pneumococcal-host interactions is most needed.
The model assumes that bacteria of different serotypes compete via direct interactions in the nasopharynx. These interactions may take the form of competition for resources, such as attachment sites or nutrients, or they may take the form of interference competition, in which a resident type produces substances toxic to other bacteria that may attempt to colonize the same host. Apart from the few studies cited above, little is known about either the intensity or the mechanisms of such inhibition. There are some epidemiologic data that indirectly indicate the existence of such competitive interactions. A study of military personnel in 1946 (27) used a very sensitive technique, mouse inoculation, to detect nasopharyngeal carriage of one or more pneumococcal serotypes. The numbers of persons carrying one, two, three, or four serotypes are given, and although the published data do not provide all of the information necessary for formal statistical inference, the pattern suggests that interference between serotypes may have occurred.
The model does not take into account acquired immunity to carriage of these bacteria, or the possibility that carriage of one serotype may inhibit future carriage of another serotype, even after the first is no longer carried. It is unclear to what degree it is realistic to ignore acquired immunity to carriage. While carriage has been shown to induce a serum antibody response in at least one report (44), it is less clear whether such responses affect carriage at the nasopharyngeal mucosa. The success of conjugate vaccines in reducing carriage indicates that some antibody responses can affect carriage. However, it remains to be demonstrated whether such responses are induced by natural exposure through the respiratory route, whether natural exposure induces responses to other, more conserved antigens or only to the capsular antigen, and whether natural exposure induces long-lived immunologic memory. Preliminary results of mathematical models that incorporate naturally acquired immunity to carriage suggest that the expected effects of vaccination on the serotype composition of the population may be different from those expected under the models described here. Therefore, further research into the microbiology and immunology of the host-bacterial relationship in the nasopharynx will be critical to understanding and predicting the population-wide effects of conjugate vaccines.
The choice of serotypes for inclusion in conjugate vaccines has been different in different locations but has generally been designed to cover serotypes that are most often implicated in invasive disease. Often, these types coincide with serotypes showing the greatest levels of antibiotic resistance (45,46). As a result, conjugate vaccination has led to a reduction in the percentage of antibiotic-resistant pneumococci carried by vaccinees (4,6).
In principle, replacement could occur with bacteria that differ from the vaccine targets not only in serotype but in species. Indeed, one of the studies of bacterial antagonism in the nasopharynx concentrated on interactions between species rather than between serotypes of the same species (30). Furthermore, even if replacement is limited to members of the same species, the serotypes that increase may tend to cause a disease different from that caused by vaccine-type organisms (e.g., otitis rather than pneumonia or bacteremia). Therefore, as conjugate vaccines are used, changes in diseases attributable to organisms that colonize the nasopharynx should be monitored.
Finally, capsular polysaccharide is not the only possible target for vaccination. Several pneumococcal vaccines based on protein antigens are in various stages of testing (47). Because these protein antigens show considerably less variation among pneumococcal isolates, vaccines based on them should be less vulnerable to serotype replacement and may be useful as complements or alternatives to polysaccharide conjugate vaccines.
The occurrence of serotype replacement in three trials of pneumococcal conjugate vaccines confirms the validity of concerns expressed in anticipation of these trials. As the results of more clinical trials become available, it will become clearer how general this phenomenon is. Mathematical models are useful in suggesting ways to improve the design of these trials and the interpretation of their results.
The extent and importance of serotype replacement will depend on many locally variable factors, the prevalence of vaccine-type organisms before vaccination, and the level of vaccine coverage. This prediction underscores the need for continuing studies of vaccination in different communities and for at least some studies in which a substantial fraction of a community receives the vaccine. Furthermore, the epidemiologic findings of these studies should be the impetus for further research into the role of serotype and other factors in determining the variation in pneumococcal virulence, the nature of immune responses to organisms like the pneumococcus at the nasopharyngeal mucosal surface, and other questions in the biology of bacterial carriage.
Dr. Lipsitch completed this research while he was a postdoctoral fellow with Dr. Bruce Levin at Emory University and a visiting scientist with Dr. George Carlone at CDC. His research includes experimental, epidemiologic, and mathematical modeling studies of vaccination and antimicrobial resistance. In September 1999, he will begin as an assistant professor of epidemiology at the Harvard School of Public Health.
Acknowledgments
The author thanks Dr. R. Moxon for introducing him to the problem of serotype replacement and Drs. K. O'Brien, B.R. Levin, O. Levine, R. Dagan, D. Stephens, M.E. Halloran, and G. Carlone for helpful discussions.
Marc Lipsitch's work is supported by grant # GM19182 from NIH.
References
- Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 1997;46:RR8.
- Centers for Disease Control and Prevention. Progress toward elimination of Haemophilus influenzae type b disease among infants and children—United States, 1987-1995. MMWR Morb Mortal Wkly Rep. 1996;45:901–6.PubMedGoogle Scholar
- Booy R, Kroll JS. Is Haemophilus influenzae finished? J Antimicrob Chemother. 1997;40:149–53. DOIPubMedGoogle Scholar
- Dagan R, Melamed R, Muallem M, Piglansky L, Greenberg D, Abramson O, Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271–8.PubMedGoogle Scholar
- Obaro SK, Adegbola RA, Banya WAS, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996;348:271–2. DOIPubMedGoogle Scholar
- Mbelle N, Wasas A, Huebner R, Kimura A, Chang I, Klugman K. Immunogenicity and impact on carriage of 9-valent pneumococcal conjugate vaccine given to infants in Soweto, South Africa. Proceedings from the Interscience Conference on Antimicrobial Agents and Chemotherapy; September 28-October 1, 1997; Toronto, Canada. LB-12, p. 13.
- Dagan R, Givon N, Yagupsky P, Porat N, Janco J, Chang I, Effect of a 9-valent pneumococcal vaccine conjugated to CRM197 (PncCRM9) on nasopharyngeal (NP) carriage of vaccine type and non-vaccine type S. pneumoniae (Pnc) strains among day-care-center (DCC) attendees. Proceedings from the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 24-27, 1998; San Diego, California. G52.
- Black S, Shinefield H, Ray P, Lewis E, Fireman B; The Kaiser Permanente Vaccine Study Group. Efficacy of heptavalent conjugate pneumococcal vaccine (Wyeth Lederle) in 37,000 infants and children: results of the Northern California Kaiser Permanente Efficacy Trial. Proceedings from the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 24-27, 1998; San Diego, California. LB-9.
- Barbour ML. Conjugate vaccines and the carriage of Haemophilus influenzae type b. Emerg Infect Dis. 1996;2:176–82. DOIPubMedGoogle Scholar
- Moxon ER. The carrier state: Haemophilus influenzae. J Antimicrob Chemother 1986;18 Suppl A:17-24.
- Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother 1986;18 Suppl A:35-45.
- Lipsitch M, Moxon ER. Virulence and transmissibility of pathogens: what is the relationship? Trends Microbiol. 1997;5:31–7. DOIPubMedGoogle Scholar
- Topley WWC. The spread of bacterial infection. Lancet 1919;July 5:1-5.
- Levin BR, Bull JJ. Short-sighted evolution and the virulence of pathogenic microbes. Trends Microbiol. 1994;2:76–81. DOIPubMedGoogle Scholar
- Barbour ML, Mayon-White RT, Coles C, Crook DWM, Moxon ER. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J Infect Dis. 1995;171:93–8.PubMedGoogle Scholar
- Adams WG, Deaver KA, Cochi SL, Plikaytis BD, Zell ER, Broome CV, Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA. 1993;269:221–6. DOIPubMedGoogle Scholar
- Wenger JD, Pierce R, Deaver K, Franklin R, Bosley G, Pigott N, Invasive Haemophilus influenzae disease: a population-based evaluation of the role of capsular polysaccharide serotype. J Infect Dis. 1992;165(Suppl 1):S34–5.PubMedGoogle Scholar
- Farley MM, Stephens DS, Brachman PS Jr. Invasive Haemophilus influenzae disease in adults: a prospective, population-based surveillance. Ann Intern Med. 1992;116:806–12.PubMedGoogle Scholar
- Nitta DM, Jackson MA, Burry VF, Olson LC. Invasive Haemophilus influenzae type f disease. Pediatr Infect Dis J. 1995;14:157–60. DOIPubMedGoogle Scholar
- Greene GR. Meningitis due to Haemophilus influenzae other than type b: case report and review. Pediatrics. 1978;62:1021–5.PubMedGoogle Scholar
- Takala AK, Eskola J, Leinonen M, Kayhty H, Nissinen A, Pekkanen E, Reduction of oropharyngeal carriage of Haemophilus influenzae type b (Hib) in children immunized with an Hib conjugate vaccine. J Infect Dis. 1991;164:982–6.PubMedGoogle Scholar
- Booy R, Heath P, Willocks L, Mayon-White D, Slack M, Moxon R. Invasive pneumococcal infections in children. Lancet. 1995;345:1245–6. DOIPubMedGoogle Scholar
- Urwin G, Krohn JA, Deaver-Robinson K, Wenger JD, Farley MM, Group HIS. Invasive disease due to Haemophilus influenzae serotype f: clinical and epidemiological characteristics in the H. influenzae serotype b vaccine era. Clin Infect Dis. 1996;22:1069–76.PubMedGoogle Scholar
- Baer M, Vuento R, Vesikari T. Increase in bacteraemic pneumococcal infections in children. Lancet. 1995;345:661. DOIPubMedGoogle Scholar
- Schuchat A, Robinson K, Wenger JD, Harrison LH, Farley M, Reingold AL, Bacterial meningitis in the United States in 1995. N Engl J Med. 1997;337:970–6. DOIPubMedGoogle Scholar
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford: Oxford University Press; 1991.
- Hodges RG, MacLeod CM, Bernhard WG. Epidemic pneumococcal pneumonia. III. Carrier studies. Am J Hyg. 1946;44:207–30.
- Pradier C, Dunais B, Carsenti-Etesse H, Largillier R, Bernard E, Dellamonica P. Nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae (PRSP): prevalence and incidence in three children's day-care centres in Nice, France, from 1994 to 1995. Proceedings from the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 15-18, 1996; New Orleans, Louisiana. C56.
- Reichler MR, Allphin AA, Breiman RF, Schreiber JR, Arnold JE, McDougal LK, The spread of multiply resistant Streptococcus pneumoniae at a day care center in Ohio. J Infect Dis. 1992;166:1346–53.PubMedGoogle Scholar
- Sanders CC, Sanders WE Jr, Harrowe DJ. Bacterial interference: effects of oral antibiotics on the normal throat flora and its ability to interfere with group A streptococci. Infect Immun. 1976;13:808–12.PubMedGoogle Scholar
- Johanson WG Jr, Blackstock R, Pierce AK, Sanford JP. The role of bacterial antagonism in pneumococcal colonization of the human pharynx. J Lab Clin Med. 1970;75:946–52.PubMedGoogle Scholar
- Venezia RA, Robertson RG. Bactericidal substance produced by Haemophilus influenzae type b. Can J Microbiol. 1975;21:1587–94. DOIPubMedGoogle Scholar
- Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci U S A. 1997;94:6571–6. DOIPubMedGoogle Scholar
- Sutton A, Schneerson R, Kendall-Morris S, Robbins JB. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982;35:95–104.PubMedGoogle Scholar
- Smith T, Lehmann D, Montgomery J, Gratten M, Riley ID, Alpers MP. Acquisition and invasiveness of different serotypes of Streptococcus pneumoniae in young children. Epidemiol Infect. 1993;111:27–39. DOIPubMedGoogle Scholar
- Ewald PW. Vaccines as evolutionary tools: the virulence-antigen strategy. In: Kaufmann SHE, editor. Concepts in vaccine development. Berlin: Walter de Gruyter; 1996.
- Moxon ER, Vaughn KA. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981;14:517–24.
- Kelly T, Dillard JP, Yother J. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect Immun. 1994;62:1813–9.PubMedGoogle Scholar
- Barnes DM, Whittier S, Gilligan PH, Soares S, Tomasz A, Henderson FW. Transmission of multidrug-resistant serotype 23F Streptococcus pneumoniae in group day care: evidence suggesting capsular transformation of the resistant strain in vivo. J Infect Dis. 1995;171:890–6.PubMedGoogle Scholar
- Takala AK, Vuopio-Varkila J, Tarkka E, Leinonen M, Musser JM. Subtyping of common pediatric pneumococcal serotypes from invasive disease and pharyngeal carriage in Finland. J Infect Dis. 1996;173:128–35.PubMedGoogle Scholar
- Gratten M, Montgomery J, Gerega G, Gratten H, Siwi H, Poli A, Multiple colonization of the upper respiratory tract of Papua New Guinea children with Haemophilus influenzae and Streptococcus pneumoniae. Southeast Asian J Trop Med Public Health. 1989;20:501–9.PubMedGoogle Scholar
- Gilks WR, Spiegelhalter DJ. A language and program for complex Bayesian modeling. Statistician. 1994;43:169–78. DOIGoogle Scholar
- Spiegelhalter DJ, Thomas A, Best NG, Gilks WR. BUGS: Bayesian inference using Gibbs sampling. Version 0.50. Cambridge: MRC Biostatistics Unit; 1995.
- Musher DM, Groover JE, Reichler MR, Riedo FX, Schwarz B, Watson DA, Emergence of antibody to capsular polysaccharides of Streptococcus pneumoniae during outbreaks of pneumonia: association with nasopharyngeal colonization. Clin Infect Dis. 1997;24:441–6.PubMedGoogle Scholar
- Dagan R, Melamed R, Muallem M, Piglansky L, Yagupsky P. Nasopharyngeal colonization in southern Israel with antibiotic-resistant pneumococci during the first 2 years of life: relation to serotypes likely to be included in pneumococcal conjugate vaccines. J Infect Dis. 1996;174:1352–5.PubMedGoogle Scholar
- Butler JC. Epidemiology of pneumococcal serotypes and conjugate vaccine formulations. Microb Drug Resist. 1997;3:125–9. DOIPubMedGoogle Scholar
- Paton JC. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 1998;6:85–7. DOIPubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 5, Number 3—June 1999
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Marc Lipsitch, Department of Epidemiology, Harvard School of Public Health, 677 Huntington Avenue, Boston, MA, 02115, USA
Top