Volume 5, Number 3—June 1999
Dispatch
New Cryptosporidium Genotypes in HIV-Infected Persons
Abstract
Using DNA sequencing and phylogenetic analysis, we identified four distinct Cryptosporidium genotypes in HIV-infected patients: genotype 1 (human), genotype 2 (bovine) Cryptosporidium parvum, a genotype identical to C. felis, and one identical to a Cryptosporidium sp. isolate from a dog. This is the first identification of human infection with the latter two genotypes.
Protozoan apicomplexan parasites from the genus Cryptosporidium infect a wide variety of hosts (1). The parasites are transmitted to humans through contaminated drinking water (2), contact with infected animals, and contact with infected persons (3). In the immunocompetent, cryptosporidiosis manifests itself as self-limited diarrhea, sometimes accompanied by nausea, abdominal cramps, fever, and vomiting. In the immunodeficient, however, cryptosporidiosis may be severe, chronic, and life-threatening (4).
Cross-infection experiments, in which Cryptosporidium oocysts were obtained from animals of one species and fed to animals of another species, have investigated the host specificity of this parasite (5). The differences observed in the host range of putative C. parvum isolates led to a proposal to establish the Cryptosporidium isolate originating from guinea pigs, morphologically indistinguishable from C. parvum, as a new species—C. wrairi—solely on the basis of experimental infection (6). The possibility of many Cryptosporidium species fostered the development of techniques suitable for typing isolates. Commonly used techniques are isoenzyme analysis (7), Western blotting (8,9), random amplified polymorphic DNA analysis (10-12), polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) analysis (13,14), and PCR followed by DNA sequencing (11,15-19).
Early studies of the polymorphism of isolates classified as C. parvum found significant geographic variation among isolates (20) in the region coding for the small subunit ribosomal RNA (SSU-rRNA), commonly used for taxonomic classification. Recently, it has been shown (21) that one of the sequences used in this analysis (22) was erroneously identified as a C. parvum sequence, while in fact it was C. muris. More recent work (e.g., Le Blancq et al. [23] and GenBank entry AF040725) has shown that the SSU-rRNA region of the C. parvum zoonotic (bovine) genotype does not show heterogeneity and is practically identical to the sequence submitted to GenBank in 1993 (accession number L16996, [24,25]).
Recently, consistent results of typing bovine and human C. parvum isolates led to unequivocal recognition of two genotypes of C. parvum. These two genotypes were reproducibly differentiated by sequencing the SSU-rRNA coding region (16), sequencing the Cryptosporidium thrombospondin-related adhesion protein (TRAP-C2) gene (17), and PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene (15). Thus far, the anthroponotic genotype (genotype 1) has been found only in infected humans, while the zoonotic genotype (genotype 2) has been found both in infected humans and in livestock, e.g., cows, lambs, goats, and horses. The published partial SSU-rRNA sequence of the C. parvum genotype 1 (16) is identical to the Cryptosporidium SSU-rRNA sequence that we observed in PCR-amplified DNA from a patient with AIDS (GenBank accession number L16997). That the data from different laboratories for these two genotypes were reproducible calls into question the recent conclusion of Tzipori and Griffiths (26) that "there are no clearly defined and fully characterized reference `strains' of Cryptosporidium."
Other new Cryptosporidium genotypes have been identified in various animals (pigs, mice, cats, and koalas) by sequencing the region coding for SSU-rRNA (16,27,28). The cat genotype is thought to represent C. felis (28). When morphologic and other data are available for these new genotypes, they may be recognized as new Cryptosporidium species.
We present results of typing Cryptosporidium isolates by direct sequencing the variable region of the SSU-rRNA amplified from fecal specimens with Cryptosporidium genus-specific diagnostic PCR primers (25). Applying this method to a set of specimens from a 3-year longitudinal study on the risk for enteric parasitosis and chronic diarrhea in immunodeficient patients with a low CD4+ count, we found the first human cases of infection by C. felis and by a newly identified zoonotic Cryptosporidium species possibly originating from a dog.
All analyzed specimens were collected from January 1991 through September 1994 in a study assessing the impact of enteric parasite-associated diarrhea in persons infected with HIV (29). Study participants answered comprehensive questionnaires concerning clinical and epidemiologic information and provided stool specimens monthly. All stool specimens were examined for C. parvum by Kinyoun carbol-fuchsin modified acid-fast stain (30) and direct immunofluorescence (31). Stained slides were examined by observing 200 oil-immersion fields. C. parvum was associated with 18 (5.1%) of the 354 acute episodes and 36 (12.9%) of the chronic episodes of diarrhea reported by the participants. All specimens from this study were preserved in separate vials of 10% formalin and polyvinyl alcohol (Para-Pak Stool System; Meridian Diagnostics, Inc., Cincinnati, OH) and thus were not suitable for molecular analysis. Some specimens were originally aliquoted and stored without preservation at -80°C. Of this set, we selected 18 available specimens for 10 randomly chosen Cryptosporidium-positive patients for this study.
An aliquot of approximately 300 µl of each stool specimen was suspended in 1 ml of 0.01 M phosphate-buffered saline, pH 7.2, containing 0.01 M of EDTA (PBS-EDTA), and the suspension was centrifuged at 14,000 x g, 4°C for 5 minutes. The pellet from this centrifugation was washed two more times under the same conditions. The pellet was resuspended in 300 µl of PBS-EDTA and used for DNA extraction, performed with the FastPrep disrupter and the FastDNA kit (BIO 101, Inc., Vista, CA) (32). Extracted DNA was stored at 4°C until PCR amplification.
Cryptosporidium genus-specific primers (CPBDIAGF and CPBDIAGR) were used to amplify the Cryptosporidium SSU-rRNA variable region (24,25). The conditions for PCR were 95°C for 15 minutes; 45 cycles of denaturation at 94°C for 30 seconds, annealing at 65°C for 30 seconds, extension at 72°C for 1 minute, 30 seconds; followed by extension at 72°C for 9 minutes; and finished with a hold step at 4°C.
PCR products were analyzed by electrophoresis on 2% SeaKem GTG agarose (Cat. No. 50074, FMC Bioproducts, Rockland, ME), stained with ethidium bromide, and visualized on UV transilluminator. The size of the diagnostic fragment amplified with these primers for C. parvum genotype 1 (GenBank accession number L16997) was 438 bp, for C. parvum genotype 2 (L16996) 435 bp, for C. baileyi (L19068) 428 bp, and for C. muris (L19069) 431 bp.
PCR products were purified by using the Wizard PCR Preps kit (Cat. No. A7170, Promega, Madison, WI). Sequencing reactions were done with the Perkin Elmer Big Dye kit (Cat. No. 4303149, PE Biosystems, Foster City, CA) and analyzed on the Perkin Elmer ABI 377 automatic DNA sequencer. Sequences were assembled by using the program SeqMan II (DNASTAR Inc., Madison, WI). Sequences were aligned with the program MACAW (33) and analyzed by programs from the PHYLIP (phylogeny inference program) package (34).
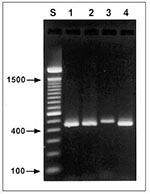
After the 18 fecal specimens were retrieved from storage and processed for PCR with the CPBDIAGF/CPBDIAGR diagnostic primer set, visualization of PCR products on 2% agarose gels found two different sizes of diagnostic bands. The size of the Cryptosporidium diagnostic bands for patients 53, 75, 119, 124, 153, 278, and 554 did not differ significantly from the size of the standard band (approximately 435 bp) obtained with cloned SSU-rRNA for the C. parvum genotype 2 (Figure 1, lane 1). On the other hand, the Cryptosporidium diagnostic bands in samples from patients 84, 184, and 485 were visibly larger, approximately 450 bp (Figure 1, lane 3 shows data for patient 84).
DNA sequence analysis showed four types of sequences (Table 1). The sequence of the diagnostic band from patient 53 was 435 bp long and was identical to the corresponding fragment of the C. parvum genotype 2 (GenBank accession number L16996) SSU-rRNA. The diagnostic bands in patients 75, 124, 153, 278, and 554 were 438 bp long and were identical to the corresponding fragment of the C. parvum genotype 1 SSU-rRNA (GenBank accession number L16997). The sequence from patient 119 was 429 bp long; the diagnostic bands from patients 84, 184, and 485 were 455 bp long and were identical to each other. Sequence similarity searches of GenBank database and molecular phylogeny analysis of the two latter types of sequences showed that, while they were not identical to any sequences in GenBank, they clustered within other representative SSU-rRNA sequences from the genus Cryptosporidium (results not shown). In addition, these sequences were significantly different from recently reported partial SSU-rRNA sequences (16,19,27,28). The 429-bp-long diagnostic fragment in patient 119 was identical to a recently identified canine C. parvum isolate SSU-rRNA sequence (GenBank accession number AF112576). The longest sequence (455 bp) from patients 84, 184, and 495 was identical in a 100-bp overlap to an updated sequence (GenBank accession number AF097430) for C. felis (also called feline C. parvum genotype) (28). Alignment of these sequences is shown in Figure 2. In the hypervariable alignment region (from position 101 to 110), all genotypes display a variable number of thymidines. Genotype 1 has the longest stretch, 11 thymidines, while the Cryptosporidium sp. (canine genotype) has only one. The sequence for C. felis has five insertions at alignment positions 45, 86, 111, 187, and 218, as well as one deletion of two thymidines at positions 197 and 198. Apart from the difference in the hypervariable region, the Cryptosporidium sp. (canine genotype) has a deletion of two bases at positions 49 and 50.
Results of genotyping the 18 specimens are in Table 2. For patients 53, 124, 153, 184, 278, and 485, only a single specimen was available. For other patients (e.g., patient 554), four specimens collected during 12 months were available. The same Cryptosporidium genotype persisted throughout a patient's infection.
Using the sequence of a diagnostic fragment of SSU-rRNA, as well as two well-established genotypes of C. parvum (anthroponotic genotype 1 and zoonotic genotype 2), we detected two new Cryptosporidium genotypes. The first, in patients 84, 184, and 485, was identical to the feline Cryptosporidium genotype (28), also described as C. felis. The second, in two specimens from patient 119, represents the newly identified Cryptosporidium sp. found in a sequence originating from a dog (GenBank accession number AF112576).
New taxons may be established within the genus Cryptosporidium on the basis of the isolate's host range together with molecular data, even though morphologic criteria are apparently lacking. We propose to use C. felis Iseki, 1979, instead of feline C. parvum genotype; we believe that the anthroponotic genotype 1 of C. parvum and the Cryptosporidium sp. (canine genotype) described here should be named as distinct species. Although the taxonomy of protists is morphology-based, the taxonomy of bacteria is being reevaluated on the basis of molecular data (35). There is no reason to use different approaches to these two kingdoms, nor is it necessary to postulate that the basis for speciation of Cryptosporidium remains ambiguous or that molecular data lend little support to separation of Cryptosporidium into distinct, valid species (26). Further, we see no evidence for the unconventional conclusions of Tzipori and Griffiths that "...genetic markers in C. parvum can change upon passage to a different host, possibly through a selective mechanism favoring different populations" (26) nor for those of Widmer (21) that "...genetic studies and experimental infections suggest that a selective mechanism triggered by a change in the intestinal environment might be involved in shaping the genetic make-up of C. parvum populations."
The finding of new Cryptosporidium genotypes in immunodeficient patients might suggest a unique susceptibility to infections by divergent Cryptosporidium species circulating in companion animals or livestock. However, recent identification of C. felis in a cow (36) may indicate a complex pattern of flow of different Cryptosporidium species in the environment. When more data on the distribution of these species become available, public health and preventive measures will need to be reevaluated, and isolates may need to be renamed to reflect their natural history. Finally, it is clear that Kinyoun carbol-fuchsin modified acid-fast stain (30) and direct immunofluorescence (31) could detect all genotypes, but this may not be true for diagnostic reagents that may be affected directly (PCR) or indirectly (Ab) by the genetic composition of the new Cryptosporidium isolates reported here. Thus, discovery of these new Cryptosporidium genotypes in human cryptosporidiosis should cause existing diagnostic tools to be reevaluated.
Dr. Pieniazek is a microbiologist with the National Center for Infectious Diseases, CDC. His research interests include molecular reference diagnosis of parasitic diseases and molecular systematics.
Acknowledgment
The authors thank Jane L. Carter for her expert help with running the DNA sequencer.
References
- Fayer R, Speer CA, Dubey JP. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton (FL): CRC Press; 1997. p. 1-41.
- MacKenzie WR, Schell WL, Blair KA, Addiss DG, Peterson DE, Hoxie NJ, Massive outbreak of waterborne Cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin Infect Dis. 1995;21:57–62.PubMedGoogle Scholar
- Navin TR. Cryptosporidiosis in humans: review of recent epidemiologic studies. Eur J Epidemiol. 1985;1:77–83. DOIPubMedGoogle Scholar
- Arrowood MJ. Diagnosis. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton (FL): CRC Press; 1997. p. 43-64.
- Lindsay DS. Laboratory models of cryptosporidiosis. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton (FL): CRC Press; 1997. p. 209-23.
- Vetterling JM, Jervis HR, Merrill TG, Sprinz H. Cryptosporidium wrairi sp. n. from the guinea pig Cavia porcellus, with an emendation of the genus. J Protozool. 1971;18:243–7.PubMedGoogle Scholar
- Awad-el-Kariem FM, Robinson HA, Dyson DA, Evans D, Wright S, Fox MT, Differentiation between human and animal strains of Cryptosporidium parvum using isoenzyme typing. Parasitology. 1995;110:129–32. DOIPubMedGoogle Scholar
- McLauchlin J, Casemore DP, Moran S, Patel S. The epidemiology of cryptosporidiosis: application of experimental sub-typing and antibody detection systems to the investigation of water-borne outbreaks. Folia Parasitol (Praha). 1998;45:83–92.PubMedGoogle Scholar
- Nichols GL, McLauchlin J, Samuel D. A technique for typing Cryptosporidium isolates. J Protozool. 1991;38:237S–40.PubMedGoogle Scholar
- Carraway M, Widmer G, Tzipori S. Genetic markers differentiate C. parvum isolates. J Eukaryot Microbiol. 1994;41:26S.PubMedGoogle Scholar
- Morgan UM, Constantine CC, O'Donoghue P, Meloni BP, O'Brien PA, Thompson RCA. Molecular characterization of Cryptosporidium isolates from humans and other animals using random amplified polymorphic DNA analysis. Am J Trop Med Hyg. 1995;52:559–64.PubMedGoogle Scholar
- Shianna KV, Rytter R, Spanier JG. Randomly amplified polymorphic DNA PCR analysis of bovine Cryptosporidium parvum strains isolated from the watershed of the Red River of the North. Appl Environ Microbiol. 1998;64:2262–5.PubMedGoogle Scholar
- Bonnin A, Fourmaux MN, Dubremetz JF, Nelson RG, Gobet P, Harly G, Genotyping human and bovine isolates of Cryptosporidium parvum by polymerase chain reaction-restriction fragment length polymorphism analysis of a repetitive DNA sequence. FEMS Microbiol Lett. 1996;137:207–11. DOIPubMedGoogle Scholar
- Widmer G, Tzipori S, Fichtenbaum CJ, Griffiths JK. Genotypic and phenotypic characterization of Cryptosporidium parvum isolates from people with AIDS. J Infect Dis. 1998;178:834–40. DOIPubMedGoogle Scholar
- Spano F, Putignani L, McLauchlin J, Casemore DP, Crisanti A. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150:209–17.PubMedGoogle Scholar
- Morgan UM, Constantine CC, Forbes DA, Thompson RCA. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J Parasitol. 1997;83:825–30. DOIPubMedGoogle Scholar
- Peng MM, Xiao L, Freeman AR, Arrowood MJ, Escalante AA, Weltman AC, Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg Infect Dis. 1997;3:567–73. DOIPubMedGoogle Scholar
- Chrisp CE, LeGendre M. Similarities and differences between DNA of Cryptosporidium parvum and C. wrairi detected by the polymerase chain reaction. Folia Parasitol (Praha). 1994;41:97–100.PubMedGoogle Scholar
- Carraway M, Tzipori S, Widmer G. Identification of genetic heterogeneity in the Cryptosporidium parvum ribosomal repeat. Appl Environ Microbiol. 1996;62:712–6.PubMedGoogle Scholar
- Kilani RT, Wenman WM. Geographical variation in 18S rRNA gene sequence of Cryptosporidium parvum. Int J Parasitol. 1994;24:303–6. DOIPubMedGoogle Scholar
- Widmer G. Genetic heterogeneity and PCR detection of Cryptosporidium parvum. Adv Parasitol. 1998;40:223–39. DOIPubMedGoogle Scholar
- Cai J, Collins MD, McDonald V, Thompson DE. PCR cloning and nucleotide sequence determination of the 18S rRNA genes and internal transcribed spacer 1 of the protozoan parasites Cryptosporidium parvum and Cryptosporidium muris. Biochim Biophys Acta. 1992;1131:317–20.PubMedGoogle Scholar
- Le Blancq SM, Khramtsov NV, Zamani F, Upton SJ, Wu TW. Ribosomal RNA gene organization in Cryptosporidium parvum. Mol Biochem Parasitol. 1997;90:463–78. DOIPubMedGoogle Scholar
- Johnson DW, Pieniazek NJ, Rose JB. DNA probe hybridization and PCR detection of Cryptosporidium parvum compared to immunofluorescence assay. Water Sci Technol. 1993;27:77–84.
- Johnson DW, Pieniazek NJ, Griffin DW, Misener L, Rose JB. Development of a PCR protocol for sensitive detection of Cryptosporidium oocysts in water samples. Appl Environ Microbiol. 1995;61:3849–55.PubMedGoogle Scholar
- Tzipori S, Griffiths JK. Natural history and biology of Cryptosporidium parvum. Adv Parasitol. 1998;40:5–36. DOIPubMedGoogle Scholar
- Morgan UM, Sargent KD, Deplazes P, Forbes DA, Spano F, Hertzberg H, Molecular characterization of Cryptosporidium from various hosts. Parasitology. 1998;117:31–7. DOIPubMedGoogle Scholar
- Sargent KD, Morgan UM, Elliot A, Thompson RCA. Morphological and genetic characterization of Cryptosporidium oocysts from domestic cats. Vet Parasitol. 1998;77:221–7. DOIPubMedGoogle Scholar
- Navin TR, Weber R, Vugia DJ, Rimland D, Roberts JM, Addiss DG, Declining CD4+ T-lymphocyte counts are associated with increased risk of enteric parasitosis and chronic diarrhea: results of a 3-year longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:154–9.PubMedGoogle Scholar
- Melvin DM, Brooke MM. Laboratory procedures for the diagnosis of intestinal parasites. 3rd ed. Atlanta: Centers for Disease Control; 1982. Publication no. (CDC) 85-8282.
- Garcia LS, Shum AC, Bruckner DA. Evaluation of a new monoclonal antibody combination reagent for the direct fluorescent detection of Giardia cysts and Cryptosporidium oocysts in human fecal specimens. J Clin Microbiol. 1992;30:3255–7.PubMedGoogle Scholar
- da Silva AJ, Bornay-Llinares FJ, Moura INS, Slemenda SB, Tuttle JL, Pieniazek NJ. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol Diagn. 1999. In press.PubMedGoogle Scholar
- Schuler GD, Altschul SF, Lipman DJ. A workbench for multiple alignment construction and analysis. Proteins. 1991;9:180–90. DOIPubMedGoogle Scholar
- Felsenstein J. PHYLIP—phylogeny inference package. Cladistics. 1989;5:164–6.
- Roth A, Fischer M, Hamid ME, Michalke S, Ludwig W, Mauch H. Differentiation of phylogenetically related slowly growing mycobacteria based on 16S-23S rRNA gene internal transcribed spacer sequences. J Clin Microbiol. 1998;36:139–47.PubMedGoogle Scholar
- Bornay-Llinares FJ, da Silva AJ, Moura IN, Myjak P, Pietkiewicz H, Kruminis-Lozowska W, Identification of Cryptosporidium felis in a cow by morphologic and molecular methods. Appl Environ Microbiol. 1999;65:1455–8.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 5, Number 3—June 1999
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Norman J. Pieniazek, Division of Parasitic Diseases, Centers for Disease Control and Prevention, 4770 Buford Highway NE, Mail Stop F13, Atlanta, GA, 30341-3724, USA; fax: 770-488-4108
Top