Malaria
CDC Yellow Book 2024
Travel-Associated Infections & DiseasesINFECTIOUS AGENT: Plasmodium spp.
ENDEMICITY
Multiple countries in Africa, the Americas, and Asia
TRAVELER CATEGORIES AT GREATEST RISK FOR EXPOSURE & INFECTION
PREVENTION METHODS
Avoid insect bites
Use malaria chemoprophylaxis
DIAGNOSTIC SUPPORT
- 770-488-7788 (M–F 9 a.m.–5 p.m. Eastern)
- 770-488-7100 (after hours)
Parasitological diagnosis: DPDx
Infectious Agent
Malaria in humans is caused by protozoan parasites of the genus Plasmodium, including Plasmodium falciparum, P. malariae, P. ovale, and P. vivax. In addition, zoonotic forms have been documented as causes of human infections and some deaths, especially P. knowlesi, a parasite of Old World (Eastern Hemisphere) monkeys, in Southeast Asia.
Transmission
Plasmodium species are transmitted by the bite of an infective female Anopheles mosquito. Occasionally, transmission occurs by blood transfusion, needle sharing, nosocomially, organ transplantation, or vertically from mother to fetus. Malaria transmission occurs in large areas of Africa, Latin America, and parts of the Caribbean, Eastern Europe, the South Pacific, and in Asia including South Asia, Southeast Asia, and the Middle East (Map 5-12, Map 5-13, and Map 5-14).
Map 5-12 Malaria-endemic destinations in the Americas & the Caribbean
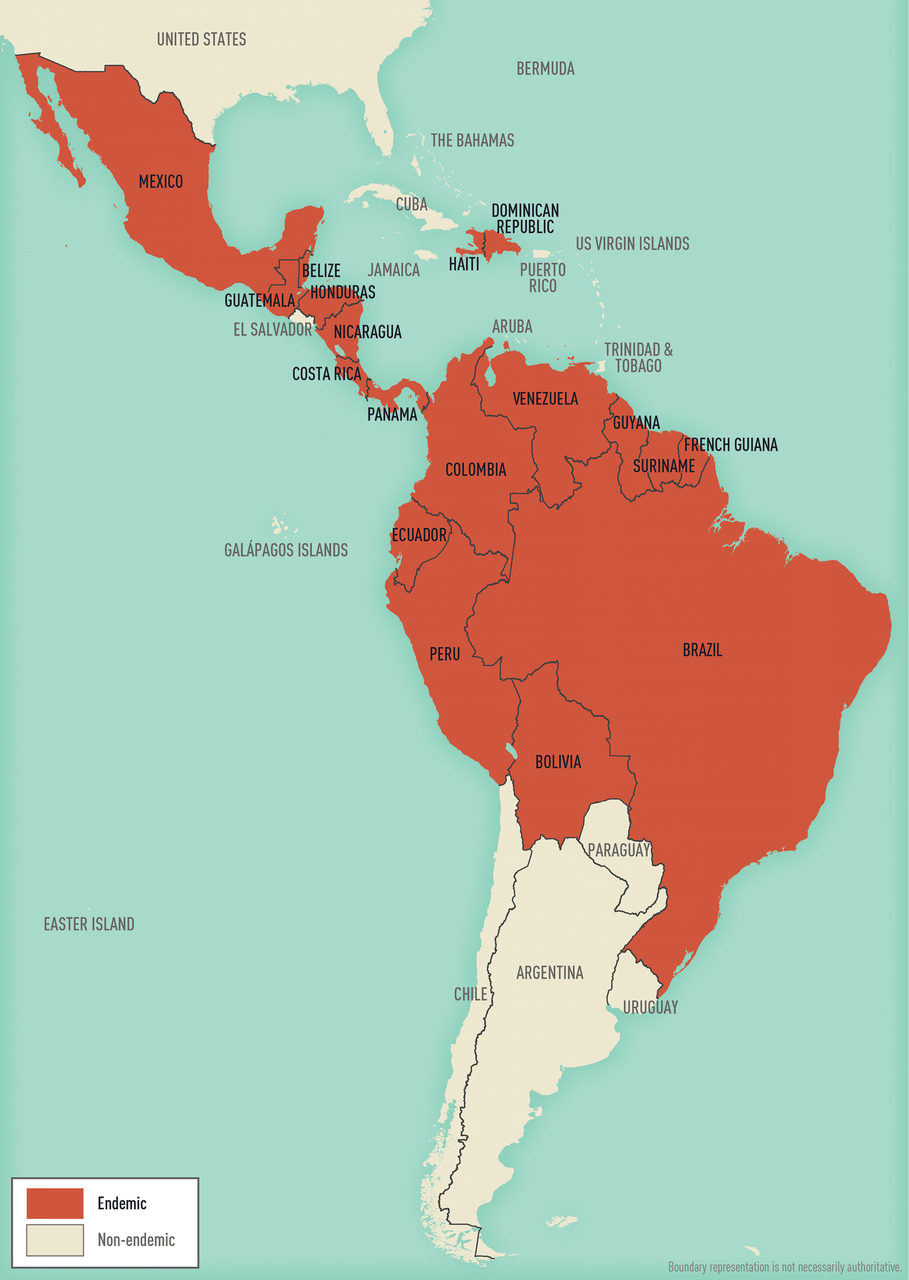
Malaria-endemic destinations are labeled using black font; destinations not endemic for malaria are labeled using gray font. Countries with areas endemic for malaria are shaded completely even if transmission occurs only in a small part of the country. For more specific within-country malaria transmission information, see Section 2, Yellow Fever Vaccine & Malaria Prevention Information, by Country.
Map 5-13 Malaria-endemic destinations in Africa & the Middle East
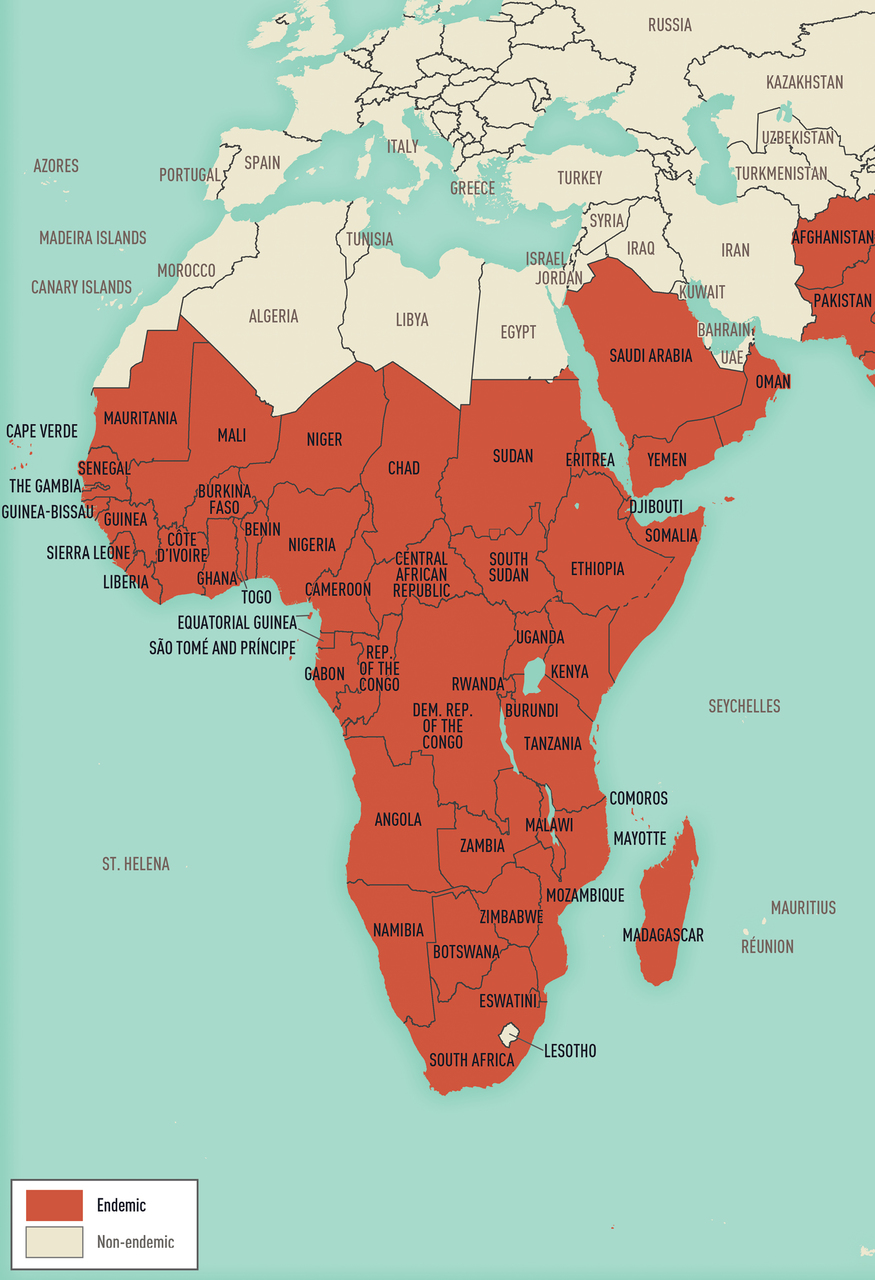
Malaria-endemic destinations are labeled using black font; destinations not endemic for malaria are labeled using gray font. Countries with areas endemic for malaria are shaded completely even if transmission occurs only in a small part of the country. For more specific within-country malaria transmission information, see Section 2, Yellow Fever Vaccine & Malaria Prevention Information, by Country.
Map 5-14 Malaria-endemic destinations in Asia & Oceania
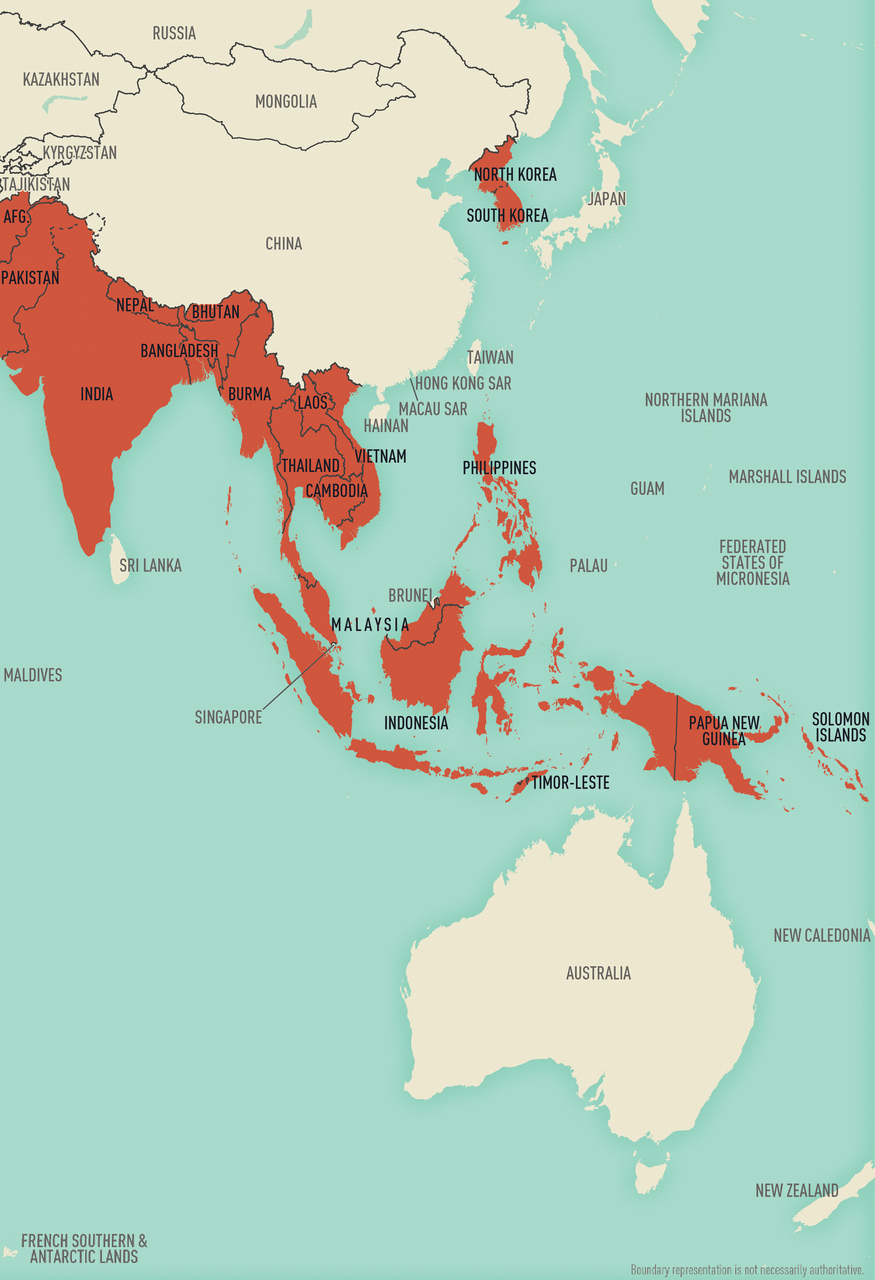
Malaria-endemic destinations are labeled using black font; destinations not endemic for malaria are labeled using gray font. Countries with areas endemic for malaria are shaded completely even if transmission occurs only in a small part of the country. For more specific within-country malaria transmission information, see Section 2, Yellow Fever Vaccine & Malaria Prevention Information, by Country.
Epidemiology
Malaria is a major international public health problem. According to the World Health Organization (WHO) World Malaria Report 2019, >90 countries reported ≈228 million infections and ≈405,000 deaths in 2018. Travelers going to malaria-endemic countries are at risk of contracting the disease, and almost all the ≈2,000 cases of malaria that occur each year in the United States are imported.
The risk of acquiring malaria differs substantially from traveler to traveler and from region to region, even within a single country. This variability is a function of the intensity of transmission within the various regions and the itinerary, duration, season, and type of travel. Risk also varies by travelers’ adherence to mosquito precautions and prophylaxis recommendations. In 2016, 2,078 cases of malaria (including 7 deaths) were diagnosed in the United States and its territories and were reported to the Centers for Disease Control and Prevention (CDC). Of cases for which country of acquisition was known, 85% were acquired in Africa, 9% in Asia, 5% in the Caribbean and the Americas, and 1% in Oceania or the Eastern Mediterranean. Of US residents with malaria who reported a reason for travel, 69% were visiting friends and relatives.
Information about malaria transmission in specific countries is derived from various sources, including WHO (see Sec. 2, Ch. 5, Yellow Fever Vaccine & Malaria Prevention Information, by Country). The information presented here was accurate at the time of publication; the risk for malaria can change rapidly and from year to year, however, because of changes in local weather conditions, mosquito vector density, and prevalence of infection. See updated information CDC website.
Clinical Presentation
Malaria is characterized by fever and influenza- like symptoms, including chills, headache, myalgias, and malaise; symptoms can occur intermittently. In severe disease, acute kidney injury, acute respiratory distress syndrome, mental confusion, seizures, coma, and death can occur. Malaria symptoms can develop as early as 7 days after being bitten by an infectious mosquito in a malaria-endemic area and as late as several months or more after exposure. Suspected or confirmed malaria, especially P. falciparum, is a medical emergency requiring urgent intervention, because clinical deterioration can occur rapidly and unpredictably. See Box 5-10 for frequently asked clinical questions.
Box 5-10 Frequently asked clinical questions
HOW DO I ADDRESS CONCERNS ABOUT SIDE EFFECTS FROM PROPHYLAXIS?
- Prophylaxis can be started earlier if the traveler has concerns about tolerating a particular medication. For example, mefloquine can be started 3–4 weeks in advance to allow potential adverse events to occur before travel. If unacceptable side effects develop, the clinician has time to change the medication before the traveler’s departure.
- The drugs used for antimalarial prophylaxis are generally well tolerated. Side effects can occur, however. Minor side effects usually do not require stopping the drug. Clinicians should determine if symptoms are related to the medicine and make a medication change if needed.
WHAT SHOULD A TRAVELER DO IF THEY MISS A DOSE OF PROPHYLAXIS?
- Compared with drugs with short half-lives, which are taken daily, drugs with longer half-lives, which are taken weekly, offer the advantage of a wider margin of error if the traveler is late with a dose.
- For a weekly drug, prophylactic blood levels can remain adequate if the dose is only 1–2 days late. If this is the case, the traveler can take a dose as soon as possible, then resume weekly doses on the originally scheduled day. If the traveler is >2 days late, blood levels might not be adequate. The traveler should take a dose as soon as possible. The weekly doses should resume at this new day of the week (the next dose is 1 week later, then weekly thereafter).
- For a daily drug, if the traveler is 1–2 days late, protective blood levels are less likely to be maintained. The traveler should take a dose as soon as possible and resume the daily schedule at the new time of day.
WHAT HAPPENS IF TOO HIGH A DOSE OF PROPHYLAXIS IS TAKEN?
- Overdose of antimalarial drugs, particularly chloroquine, can be fatal. Medications should be stored in childproof containers out of reach of infants and children.
ISN’T MALARIA A TREATABLE DISEASE? WHY NOT CARRY A TREATMENT DOSE OF ANTIMALARIALS INSTEAD OF TAKING MALARIA PROPHYLAXIS?
- Malaria could be fatal even when treated, which is why prevention is always preferable to treating infections after they occur.
WHAT SHOULD BE DONE IF FEVER DEVELOPS WHILE TRAVELING IN A MALARIA-ENDEMIC AREA?
- Malaria and other potentially life-threatening infections acquired during travel could be fatal if treatment is delayed. Travelers should promptly seek medical help and continue to take malaria prophylaxis while in the malaria-endemic area.
WHAT SHOULD BE DONE IF A TRAVELER WHO TOOK MALARIA PROPHYLAXIS DEVELOPS FEVER AFTER RETURNING FROM THEIR TRIP?
- Malaria prophylaxis, while highly effective, is not 100% effective. Travelers should be advised to seek medical care immediately if fever develops, report their travel history, get tested for malaria, and get treated promptly if infection is confirmed.
- Malaria smear or a rapid diagnostic test must be performed, and results obtained immediately (within a few hours). These tests should not be sent out to reference laboratories that take days to weeks to return results. Empiric treatment with antimalarial drugs is not recommended because the malaria smear provides critical information for appropriate treatment. If a patient has an illness suggestive of severe malaria and a compatible travel history in an area where malaria transmission occurs, and malaria testing is not immediately available, start treatment as soon as possible, even before the diagnosis is established. See CDC recommendations for malaria treatment.
Diagnosis
Travelers with symptoms of malaria should seek medical evaluation as soon as possible, even if still traveling. Consider malaria in any patient with a febrile illness who has recently returned from a malaria-endemic country. Diagnostic assistance is available from state public health laboratories or CDC. The CDC malaria laboratory can assist in speciating malaria by blood smear microscopy, or confirm species by PCR testing. The CDC laboratory also can assess malaria parasites for mutations that confer resistance to medications. Serologic testing, used in certain situations (e.g., case investigations), can also be done by CDC laboratories.
In the United States, malaria is a notifiable disease. Health care providers must report cases of malaria diagnosed via microscopy or PCR in the United States and its territories to local or state health departments. See more information on reporting malaria.
Blood Smear Microscopy
Blood smear microscopy remains the most important method for malaria diagnosis. Microscopy can provide immediate information about the presence of parasites, allow quantification of the density of the infection, and allow determination of the species of the malaria parasite—all of which are necessary for providing the most appropriate treatment. Tests should be performed immediately when ordered by a health care provider, and microscopy results should be available as soon as possible, ≤24 hours of the patient’s presentation. Assistance with speciation of malaria on smears is available from state health departments or CDC.
In resource-limited settings, and particularly in sub-Saharan Africa, overdiagnosis and the rate of false-positive microscopy for malaria can be high; warn travelers that a local diagnosis of malaria could be incorrect. In such cases, acutely ill travelers should seek the best available medical services and continue their prophylaxis regimen until they have a definitive diagnosis.
Rapid Diagnostic Testing
Rapid diagnostic tests (RDTs) for malaria detect antigens derived from malaria parasites. Malaria RDTs are immunochromatographic tests that most often use a dipstick or cassette format and provide results in 2–15 minutes. RDTs offer a useful alternative to microscopy in situations where reliable microscopic diagnosis is not immediately available. Although RDTs can detect malaria antigens within minutes, they have several limitations. RDTs cannot distinguish between all Plasmodium species that affect humans, they might be less sensitive than expert microscopy or PCR for diagnosis, they cannot quantify parasitemia, and an RDT-positive test result might persist for days or weeks after an infection has been treated and cleared. Thus, RDTs are not useful for assessing response to therapy. Furthermore, in some areas, mutations are increasingly being observed in malaria parasites, resulting in an absence of the malaria antigen usually detected by many RDTs, including the only RDT used in the United States. The absence of this parasite antigen in peripheral blood can lead to false-negative RDT test results.
Both positive and negative RDT results must always be confirmed by microscopy. Microscopy confirmation of the RDT result should occur as soon as possible, because the information on the presence, density, and parasite species is critical for optimal management of malaria. The US Food and Drug Administration (FDA) has approved an RDT (the BinaxNOW Malaria test) for hospital and commercial laboratory use; the test is not approved for use by clinicians or patients. Laboratories that do not provide in-house, on-the-spot microscopy services should maintain a stock of malaria RDTs so that they will be able to perform immediate malaria diagnostic testing when needed.
PCR Testing
PCR tests also are available to detect malaria parasites. These tests are more sensitive than routine microscopy, but results are not usually available as quickly as microscopy results, thus limiting the utility of PCR for acute diagnosis and initial clinical management. Use of PCR testing is encouraged to confirm the species of malaria parasite and detect mixed infections.
Treatment
Malaria can be treated effectively if treatment begins early in the disease; delaying therapy, however, can have serious or even fatal consequences. Specific treatment options depend on the species of malaria, the severity of infection, the likelihood of drug resistance (based on where the infection was acquired), the patient’s age, and whether the patient is pregnant or breastfeeding.
See detailed CDC recommendations for malaria treatment. For assistance with the diagnosis or treatment of malaria, call the CDC Malaria Hotline (770-488-7788 or toll-free at 855-856-4713) from 9 a.m. to 5 p.m. Eastern Time. After hours, on weekends, or on holidays, call the CDC Emergency Operations Center at 770-488-7100 and ask the operator to contact the subject matter expert on call for the Malaria Branch. In addition, consult a clinician specializing in travel or tropical medicine or infectious diseases.
Travelers who decline to take prophylaxis, who choose a suboptimal drug regimen (e.g., chloroquine in an area with chloroquine-resistant P. falciparum), or who require a less-than-optimal drug regimen for medical reasons are at increased risk for acquiring malaria and then needing prompt treatment while abroad. Medications not used in the United States to treat malaria (e.g., halofantrine, sulfadoxine-pyrimethamine) are widely available abroad. CDC does not recommend halofantrine for treatment because of documented adverse cardiac events, including deaths. These adverse events have occurred in people with and without preexisting cardiac problems, and both in the presence and absence of other antimalarial drugs. Sulfadoxine-pyrimethamine is not recommended because of widespread drug-resistant Plasmodium.
Reliable Supply of Malaria Treatment
Some travelers who take effective prophylaxis but who will be in remote areas might decide, in consultation with their travel health provider, to also carry a reliable supply of a full course of an approved malaria treatment regimen. In the event a traveler carrying a reliable supply is diagnosed with malaria, they will have immediate access to an approved treatment.
CDC recommends that the reliable supply be acquired in the United States, so clinicians can consider the traveler’s other medical conditions or medications when selecting an antimalarial drug and to avoid the possibility of travelers obtaining counterfeit drugs in the local pharmacy or market, or depleting local resources. In rare instances when access to medical care is not available and the traveler develops a febrile illness consistent with malaria, the reliable supply medication can be self-administered presumptively. Advise travelers that self-treatment of a possible malarial infection is only a temporary measure, and that prompt medical evaluation is imperative.
Two malaria treatment regimens available in the United States can be prescribed as a reliable supply for self-treatment: atovaquone-proguanil and artemether-lumefantrine. To treat malaria, CDC recommends against using the same (or related) drug that has been taken for prophylaxis. For example, atovaquone-proguanil can be used as a reliable supply medication by travelers who are not taking atovaquone-proguanil for prophylaxis. See Table 5-26 for dosing recommendations.
Table 5-26 Reliable supply regimens for malaria treatment1
DRUG
ADULT DOSE
PEDIATRIC DOSE
COMMENTS
DRUG
ATOVAQUONE-PROGUANIL2
Adult tablets:
- Atovaquone 250 mg
- Proguanil 100 mg
Pediatric tablets:
- Atovaquone 62.5 mg
- Proguanil 25 mg
ADULT DOSE
4 adult tablets taken orally (as a single daily dose) for 3 consecutive days
PEDIATRIC DOSE
Weight-based daily dose taken orally (as a single daily dose) for 3 consecutive days
5–8 kg: 2 pediatric tablets
9–10 kg: 3 pediatric tablets
11–20 kg: 1 adult tablet
21–30 kg: 2 adult tablets
31–40 kg: 3 adult tablets
>41 kg: 4 adult tablets
COMMENTS
Contraindicated in people with severe renal impairment (creatinine clearance <30 mL/min).
Not recommended for people taking atovaquone-proguanil prophylaxis.
Not recommended for children weighing <5 kg, or women who are pregnant or breastfeeding infants weighing <5 kg.
DRUG
ARTEMETHER-LUMEFANTRINE2
One tablet
- Artemether 20 mg
- Lumefantrine 120 mg
ADULT & PEDIATRIC DOSE
Weight-based treatment schedule for both adult and pediatric patients. Patients should take an initial dose, followed by a second dose 8 hours later, then 1 dose twice a day for the next 2 days (total of 6 oral doses over 3 days).
5 kg to <15 kg: 1 tablet per dose
15 kg to <25 kg: 2 tablets per dose
25 kg to <35 kg: 3 tablets per dose
≥35 kg: 4 tablets per dose
COMMENTS
Not recommended for people taking mefloquine prophylaxis.
Not recommended for children weighing <5 kg, or women breastfeeding infants weighing <5 kg.
1A reliable supply is a complete course of an approved malaria treatment regimen obtained in the United States before travel. A reliable supply is not counterfeit or substandard; will not interact adversely with the patient’s other medicines, including malaria chemoprophylaxis; will not deplete local resources in the destination country.
2If used for presumptive self-treatment, patients should seek medical care as soon as possible.
Prevention
Malaria prevention consists of a combination of mosquito avoidance measures and chemoprophylaxis. Prevention measures must address all malaria species in the travel area and apply to both short-term and long-term travelers. Although highly efficacious, interventions are not 100% effective, so all febrile persons returning from malaria-endemic areas should be tested for malaria even if they took chemoprophylaxis.
Preventing malaria involves striking a balance between effectiveness and safety: ensuring that all people at risk for infection use the recommended prevention measures, and preventing rare occurrences of adverse effects. Conduct an individual risk assessment for every traveler by collecting a detailed travel itinerary, including countries, specific areas to be visited in those countries (e.g., cities, rural areas, both), types of accommodation, season, and style of travel. Modify the risk assessment depending on traveler characteristics (e.g., pregnancy, underlying health conditions) and malaria characteristics at the destination (e.g., intensity of transmission, local parasite resistance to drugs). Depending on the level of risk, it might be appropriate to recommend no specific interventions, mosquito avoidance measures only, or mosquito avoidance measures plus chemoprophylaxis.
Several factors increase a traveler’s risk for malaria. Travel, even for short periods of time, to areas with intense malaria transmission can result in infection. Malaria transmission is not distributed homogeneously throughout a country, so review the exact itinerary to determine if travel will occur in highly endemic areas. In countries where malaria is seasonal, travel during peak transmission season also increases risk. Travelers going to rural areas or staying in accommodations without screens or air conditioning also will be at greater risk. The greatest risk for malaria is associated with first- and second-generation immigrants living in nonendemic countries who return to their countries of origin to visit friends and relatives (VFRs). VFR travelers might perceive themselves to be at no risk because they grew up in a malaria-endemic country and consider themselves immune to the disease. Tolerance acquired through continuous exposure to malaria is quickly lost, however; consider VFRs to have the same risk as other nonimmune travelers (see Sec. 9, Ch. 9, Visiting Friends & Relatives: VFR Travel). Also remind travelers that they could become infected even if they had malaria before, and they still need to take preventive measures.
Mosquito Avoidance Measures
Because of the nocturnal feeding habits of Anopheles mosquitoes, malaria transmission occurs primarily between dusk and dawn. Travelers can reduce contact with mosquitoes by remaining in enclosed air-conditioned rooms or well-screened areas, sleeping under mosquito nets (preferably insecticide-treated), using an effective insecticide spray or mosquito coils in living and sleeping areas during evening and nighttime hours, and wearing clothes that cover most of the body.
All travelers should use an effective mosquito repellent, such as those that contain DEET (see Sec. 4, Ch. 6, Mosquitoes, Ticks & Other Arthropods). Repellents should be applied to exposed parts of the skin. If travelers are also wearing sunscreen, they should apply sunscreen first and insect repellent second. In addition to using a topical insect repellent, a permethrin-containing product can be applied to mosquito nets and clothing for additional protection against mosquitoes. Mosquito repellant–impregnated clothing also is available.
Chemoprophylaxis
Choosing a Drug to Prevent Malaria
All recommended primary prophylaxis regimens involve taking a medicine before, during, and after travel to an area with malaria. Beginning the drug before travel allows the antimalarial agent to be in the blood before the traveler is exposed to malaria parasites. In choosing a prophylaxis regimen before travel, the traveler and the travel health provider should consider several factors, including the presence of antimalarial drug resistance in the area of travel, length of travel, the patient’s other medical conditions, allergy history, other medications prescribed or already being taken (to assess possible drug interactions), potential side effects, and the cost of the antimalarial. Long-term travelers, defined as people who travel for ≥6 months, have additional considerations (see Box 5-11). Table 5-27 lists some of the benefits and limitations of medicines used for malaria prophylaxis; see additional information about choosing a malaria prophylaxis regimen.
Recommendations for drugs to prevent malaria by country of travel can be found in Sec. 2, Ch. 5, Yellow Fever Vaccine & Malaria Prevention Information, by Country. Recommended drugs for each country are listed in alphabetical order and have comparable efficacy in that country. When >1 drug is recommended, Table 5-27 can help with the decision-making process. No antimalarial drug is 100% protective; therefore, travelers must combine prophylaxis with mosquito avoidance and personal protective measures (e.g., insect repellent, long sleeves, long pants, sleeping in a mosquito-free setting, using an insecticide-treated mosquito net).
Table 5-27 Malaria chemoprophylaxis: prescribing considerations
DRUG
REASONS TO CONSIDER USING THIS DRUG
REASONS TO CONSIDER AVOIDING THIS DRUG
DRUG
ATOVAQUONE-PROGUANIL
REASONS TO CONSIDER USING THIS DRUG
Good for last-minute travelers because the drug is started 1–2 days before travel.
Some people prefer to take a daily medicine.
Good choice for shorter trips because the traveler takes the medicine for only 7 days after leaving malaria-endemic area, rather than for 4 weeks.
Well tolerated and side effects uncommon.
Pediatric tablets are available and might be more convenient.
REASONS TO CONSIDER AVOIDING THIS DRUG
Cannot be used by women who are pregnant or who are breastfeeding a child that weighs <5 kg.
Cannot be taken by people with severe renal impairment.
Tends to be more expensive than some of the other options, especially for long trips.
Some people (including children) would rather not take medicine every day.
DRUG
CHLOROQUINE
REASONS TO CONSIDER USING THIS DRUG
Some people would rather take medicine weekly.
Good choice for long trips because it is taken only weekly.
Some people are already taking hydroxychloroquine chronically for rheumatologic conditions; in those instances, they might not have to take an additional medicine.
Can be used in all trimesters of pregnancy.
REASONS TO CONSIDER AVOIDING THIS DRUG
Cannot be used in areas with chloroquine or mefloquine resistance.
Can exacerbate psoriasis.
Some people would rather not take a weekly medication.
For short trips, some people would rather not take medication for another 4 weeks after leaving malaria-endemic areas.
Not a good choice for last-minute travelers, because drug needs to be started 1–2 weeks before travel.
DRUG
DOXYCYCLINE
REASONS TO CONSIDER USING THIS DRUG
Some people prefer to take a daily medicine.
Good for last-minute travelers because the drug is started 1–2 days before travel.
Tends to be the least expensive antimalarial drug.
People already taking doxycycline chronically to prevent acne do not have to take an additional medicine.
Doxycycline also can prevent other infections (e.g., rickettsial infections, leptospirosis); thus, might be preferred by people planning to camp, hike, and swim in fresh water where risk is high
REASONS TO CONSIDER AVOIDING THIS DRUG
Cannot be used by women who are pregnant or who are breastfeeding a child, or by children aged <8 years.
Some people would rather not take medicine every day.
For short trips, some people would rather not take medication for another 4 weeks after leaving malaria-endemic areas.
People prone to getting vaginal yeast infections when taking antibiotics might prefer taking a different medicine.
People might want to avoid the increased risk of sun sensitivity.
Some people are concerned about the potential of getting an upset stomach from doxycycline.
DRUG
MEFLOQUINE
REASONS TO CONSIDER USING THIS DRUG
Some people would rather take medicine weekly.
Good choice for long trips because it is taken only weekly.
Can be used in all trimesters of pregnancy and during breastfeeding.
REASONS TO CONSIDER AVOIDING THIS DRUG
Cannot be used in areas with mefloquine-resistant Plasmodium spp.
Cannot be used in patients with certain psychiatric conditions; some travelers without psychiatric conditions would prefer not taking a medication with known neuropsychiatric side effects.
Cannot be used in patients with a seizure disorder.
Not recommended for people with cardiac conduction abnormalities.
Not a good choice for last-minute travelers because drug needs to be started ≥2 weeks before travel.
Some people would rather not take a weekly medication.
For short trips, some people would rather not take medication for another 4 weeks after leaving malaria-endemic areas.
DRUG
PRIMAQUINE
REASONS TO CONSIDER USING THIS DRUG
One of the most effective drugs for prevention of P. vivax; thus, a good choice for travel to places with >90% P. vivax.
Good choice for shorter trips because the traveler takes the medicine for 7 days after leaving a malaria-endemic area, rather than for 4 weeks.
Good for last-minute travelers because the drug is started 1–2 days before travel.
Some people prefer to take a daily medicine.
REASONS TO CONSIDER AVOIDING THIS DRUG
Cannot be used in patients with G6PD deficiency.
Cannot be used in patients who have not been tested for G6PD deficiency.
Costs and delays associated with getting a quantitative G6PD test might prohibit testing; however, the test only has to be done once. After a normal G6PD level is verified and documented, the test does not have to be repeated the next time primaquine or tafenoquine is considered.
Cannot be used by women who are pregnant.
Cannot be used by women who are breastfeeding unless the infant has also been tested for G6PD deficiency.
Some people (including children) would rather not take medicine every day.
Some people are concerned about the potential of getting an upset stomach from primaquine.
DRUG
TAFENOQUINE
REASONS TO CONSIDER USING THIS DRUG
One of the most effective drugs for prevention of P. vivax malaria but also prevents P. falciparum.
Good choice for shorter trips because the traveler takes the medicine once, 1 week after leaving malaria-endemic area, rather than for 4 weeks.
Good for last-minute travelers because the drug is started 3 days before travel.
REASONS TO CONSIDER AVOIDING THIS DRUG
Cannot be used in people with G6PD deficiency.
Cannot be used in patients who have not been tested for G6PD deficiency.
Costs and delays associated with getting a quantitative G6PD test might prohibit testing; however, the test only has to be done once. After a normal G6PD level is verified and documented, the test does not have to be repeated the next time tafenoquine or primaquine is considered.
Cannot be used by children.
Cannot be used by women who are pregnant.
Cannot be used by women who are breastfeeding unless the infant has also been tested for G6PD deficiency.
Not recommended for patients with psychotic disorders.
Abbreviations: G6PD, glucose-6-phosphate-dehydrogenase
Box 5-11 Malaria prevention & prophylaxis considerations for the long-term traveler (travel >6 months)
CONSIDERATIONS
- Malaria prevention measures are the same for both short- and long-term travelers.
- Longer stays mean longer duration of exposure and increased risk of acquiring malaria.
- Travelers’ attention to mosquito avoidance can wane over time.
- Travelers might not adhere to a lengthy course of malaria prophylaxis due to forgetfulness, fear of side effects, and the possible declining sense of risk and need over time.
- Travelers might move between highly endemic or low endemic areas within a country or region.
- Travelers might have a decreased sense of risk and concern about malaria after engaging in local conversations and lore, particularly regarding malaria immunity over time.
- Travelers who become ill with malaria in countries with limited access and quality of health care might not receive appropriate or effective treatment.
ADDITIONAL ADVICE FOR LONG-TERM TRAVELERS
- Travelers should not count on being able to obtain safe, reliable malaria prophylaxis medication abroad; strongly advise that before leaving the United States they purchase enough medication to last them for the entire duration of their travel to malaria-endemic areas.
- Emphasize continued adherence to and safety of malaria prophylaxis drugs.
- Develop a plan for seeking immediate care when ill with fever, including where to get promptly tested and treated for malaria.
- Advise travelers to purchase travel insurance, including contingencies for medical evacuation.
- Consider having a reliable supply of a treatment dose of antimalarial drugs available in case malaria is diagnosed while traveling.
Medications Used for Prophylaxis
Atovaquone-Proguanil
Atovaquone-proguanil (Malarone) is a fixed combination of the drugs atovaquone and proguanil. Prophylaxis should begin 1–2 days before travel to malaria-endemic areas; the medication should then be taken daily, at the same time each day, while in the malaria-endemic areas, and daily for 7 days after leaving the endemic areas (see Table 5-28 for recommended dosages). Atovaquone-proguanil is well tolerated, and side effects are rare. The most common adverse effects reported in people using atovaquone-proguanil for prophylaxis or treatment are abdominal pain, nausea, vomiting, and headache.
Atovaquone-proguanil is not recommended for prophylaxis in children weighing <5 kg (11 lb), pregnant women, women breastfeeding infants <5 kg, or patients with severe renal impairment (creatinine clearance <30 mL/min). Proguanil can increase the effect of warfarin, so travelers might need international normalized ratio monitoring or adjustment of warfarin dosage. No data are available, however, regarding the clinical impact of taking atovaquone-proguanil and warfarin at the same time.
Table 5-28 Malaria chemoprophylaxis: dosing information
DRUG
INDICATIONS
ADULT DOSE
PEDIATRIC DOSE
DOSING / CONTRAINDICATIONS / PRECAUTIONS
DRUG
ATOVAQUONE-PROGUANIL
INDICATIONS
Prophylaxis in all malaria-endemic areas
ADULT DOSE
Adult tablets:
- Atovaquone 250 mg
- Proguanil 100 mg
1 adult tablet taken orally, 1×/day
PEDIATRIC DOSE
Pediatric tablets:
- Atovaquone 62.5 mg
- Proguanil 25 mg
Weight-based daily dosing schedule (taken orally, 1×/day)
5 kg to <8 kg: 1/2 pediatric tablet
8 kg to <10 kg: 3/4 pediatric tablet
10 kg to <20 kg: 1 pediatric tablet
20 kg to <30 kg: 2 pediatric tablets
30 kg to <40 kg: 3 pediatric tablets
≥40 kg: 1 adult tablet
DOSING / CONTRAINDICATIONS / PRECAUTIONS
Begin taking 1–2 days before travel to malaria-endemic areas.
Take 1×/day, at the same time each day, while in malaria-endemic areas. Continue taking 1×/day for an additional 7 days after leaving endemic areas.
Contraindicated in people with severe renal impairment (creatinine clearance <30 mL/min).
Take with food or a milky drink.
Not recommended for children weighing <5 kg, or women who are pregnant or breastfeeding infants weighing <5 kg.
A pharmacist might need to prepare and dispense partial tablet doses in individual capsules, as described in the text.
DRUG
CHLOROQUINE
INDICATIONS
Prophylaxis only in areas with chloroquine-sensitive malaria
ADULT DOSE
300 mg base (500 mg salt) taken orally, once a week
PEDIATRIC DOSE
5 mg/kg base (8.3 mg/kg salt), up to a maximum dose of 300 mg base (500 mg salt), taken orally, 1×/week
DOSING / CONTRAINDICATIONS / PRECAUTIONS
Begin taking 1–2 weeks before travel to malaria-endemic areas.
Take 1×/week, on the same day each week, while in malaria-endemic areas.
Continue taking 1×/week for another 4 weeks after leaving endemic areas.
Can exacerbate psoriasis.
DRUG
DOXYCYCLINE
INDICATIONS
Prophylaxis in all malaria-endemic areas
ADULT DOSE
100 mg taken orally, 1×/day
PEDIATRIC DOSE
≥8 years of age: 2.2 mg/kg, up to a maximum dose of 100 mg, taken orally, 1×/day
DOSING / CONTRAINDICATIONS / PRECAUTIONS
Begin taking 1–2 days before travel to malaria-endemic areas.
Take 1×/day, at the same time each day, while in malaria-endemic areas. Continue taking 1×/day for another 4 weeks after leaving endemic areas.
Contraindicated in children aged <8 years and in women who are pregnant.
DRUG
HYDROXY-CHLOROQUINE
INDICATIONS
An alternative to chloroquine for prophylaxis only in areas with chloroquine-sensitive malaria
ADULT DOSE
310 mg base (400 mg salt) taken orally, 1×/week
PEDIATRIC DOSE
5 mg/kg base (6.5 mg/kg salt), up to a maximum dose of 310 mg base (400 mg salt), taken orally, 1×/week
DOSING / CONTRAINDICATIONS / PRECAUTIONS
Begin taking 1–2 weeks before travel to malaria-endemic areas.
Take 1×/week, on the same day each week, while in malaria-endemic areas.
Continue taking 1×/week for another 4 weeks after leaving endemic areas.
DRUG
MEFLOQUINE
INDICATIONS
Prophylaxis in areas with mefloquine-sensitive malaria
ADULT DOSE
228 mg base (250 mg salt) taken orally, 1×/week
PEDIATRIC DOSE
Weight-based weekly dosing schedule (taken orally, 1×/week)
≤9 kg: 4.6 mg/kg base (5 mg/kg salt)
>9–19 kg: 1/4 tablet
>19–30 kg: 1/2 tablet
>30–45 kg: 3/4 tablet
>45 kg: 1 tablet
DOSING / CONTRAINDICATIONS / PRECAUTIONS
Begin taking ≥2 weeks before travel to malaria-endemic areas.
Take 1×/week, on the same day each week, while in malaria-endemic areas.
Continue taking 1×/week for another 4 weeks after leaving endemic areas.
Contraindicated in people allergic to mefloquine or related compounds (quinidine, quinine) and in people with active depression, a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia, other major psychiatric disorders, or seizures.
Use with caution in people with psychiatric disturbances or a previous history of depression.
Not recommended for people with cardiac conduction abnormalities.
DRUG
PRIMAQUINE1
INDICATIONS
Prophylaxis for short-duration travel to areas with principally P. vivax.
Terminal prophylaxis (presumptive antirelapse therapy) to decrease the risk for relapses of P. vivax and P. ovale.
ADULT DOSE
30 mg base (52.6 mg salt) taken orally, 1×/day.
Same dose used for both primary and terminal prophylaxis; duration of therapy differs.
PEDIATRIC DOSE
0.5 mg/kg base (0.8 mg/kg salt), up to maximum dose of 30 mg base (52.6 mg salt), taken orally, 1×/day
Same dose for used both primary and terminal prophylaxis; duration of therapy differs.
DOSING / CONTRAINDICATIONS / PRECAUTIONS
Begin taking 1–2 days before travel to malaria-endemic areas.
Take 1×/day, at the same time each day, while in malaria-endemic areas. Continue taking 1×/day for an additional 7 days after leaving endemic areas.
Terminal prophylaxis indicated for people with prolonged exposure to P. ovale, P. vivax, or both. Take daily for 14 days after departure from the malaria-endemic area.
Contraindicated in people with G6PD deficiency.
Also contraindicated during pregnancy and breastfeeding unless the breastfed infant has a documented normal G6PD level.
DRUG
TAFENOQUINE1
INDICATIONS
Prophylaxis in all malaria-endemic areas
ADULT DOSE
200 mg orally
PEDIATRIC DOSE
Not indicated for use in children
DOSING / CONTRAINDICATIONS / PRECAUTIONS
Begin taking 3 days before travel to malaria-endemic areas.
Take 1×/week, on the same day each week, while in malaria-endemic areas. Take 1 additional dose 1 week after leaving endemic areas.
Contraindicated in people with G6PD deficiency.
Also contraindicated during pregnancy and breastfeeding unless the breastfed infant has a documented normal G6PD level.
Abbreviations: G6PD, glucose-6-phosphate-dehydrogenase
1Before prescribing primaquine or tafenoquine to any patient, document a normal G6PD level using a quantitative test.
Chloroquine & Hydroxychloroquine
Chloroquine phosphate or hydroxychloroquine sulfate (Plaquenil) can be used to prevent malaria only in destinations where chloroquine-resistant Plasmodium spp. are not active (see Sec. 2, Ch. 5, Yellow Fever Vaccine & Malaria Prevention Information, by Country). Prophylaxis should begin 1–2 weeks before travel to malaria-endemic areas. Travelers should continue taking the drug once a week, on the same day of the week, during travel in malaria-endemic areas, and for 4 weeks after they leave endemic areas (see Table 5-28 for recommended dosages).
Reported side effects of chloroquine and hydroxychloroquine include blurred vision, dizziness, gastrointestinal disturbance, headache, insomnia, and pruritus, but generally, these effects do not require travelers to discontinue the drug. High doses of chloroquine (e.g., those used to treat rheumatoid arthritis) have been associated with retinopathy; this serious side effect appears to be extremely unlikely when chloroquine is used for routine weekly malaria prophylaxis. Chloroquine and related compounds reportedly can exacerbate psoriasis. People who experience uncomfortable side effects after taking chloroquine might tolerate the drug better by taking it with meals. As an alternative, a traveler experiencing side effects might better tolerate the related compound, hydroxychloroquine sulfate.
Doxycycline
Doxycycline prophylaxis should begin 1–2 days before travel to malaria-endemic areas. Doxycycline should then be taken once a day, at the same time each day, during travel in malaria-endemic areas and daily for 4 weeks after the traveler leaves endemic areas. Insufficient data exist on the antimalarial prophylactic efficacy of related compounds (e.g., minocycline, commonly prescribed for the treatment of acne). People on a long-term regimen of minocycline who need malaria prophylaxis should stop taking minocycline 1–2 days before travel and start doxycycline instead. Minocycline can be restarted after the full course of doxycycline is completed (see Table 5-28 for recommended dosages).
Doxycycline can cause photosensitivity, usually manifested as an exaggerated sunburn reaction. The risk for such a reaction can be minimized by avoiding prolonged, direct exposure to the sun and by using sunscreen (see Sec. 4, Ch. 1, Sun Exposure). In addition, doxycycline use is associated with an increased frequency of vaginal yeast infections.
Gastrointestinal side effects (nausea, vomiting) can be minimized by taking the drug with a meal or by specifically prescribing doxycycline monohydrate or the enteric-coated doxycycline hyclate, rather than the generic doxycycline hyclate, which is often less expensive. To reduce the risk for esophagitis, advise travelers to swallow the medicine with sufficient fluids and to avoid taking doxycycline shortly before going to bed.
Doxycycline is contraindicated in people with an allergy to tetracyclines, in pregnant women, and in infants and children aged <8 years. Vaccination with the oral typhoid vaccine Ty21a should be completed ≥24 hours before taking a dose of doxycycline.
Mefloquine
Mefloquine prophylaxis should begin ≥2 weeks before travel to malaria-endemic areas. Travelers should continue taking the drug weekly, on the same day each week, during travel in malaria-endemic areas and for 4 weeks after leaving endemic areas (see Table 5-28 for recommended dosages).
At prophylactic doses, mefloquine has been associated with rare but serious adverse reactions (e.g., psychosis, seizures); these reactions are more frequent with the higher doses used for treatment. Other side effects reported in prophylaxis studies include abnormal dreams, anxiety disorder, depression, dizziness, gastrointestinal disturbance, headache, insomnia, and visual disturbances. Other neuropsychiatric disorders occasionally reported include aggressive behavior, agitation or restlessness, confusion, encephalopathy, forgetfulness, hallucinations, mood changes, panic attacks, paranoia, and sensory and motor neuropathies (e.g., ataxia, paresthesia, tremors). On occasion, psychiatric symptoms have been reported to continue long after mefloquine has been stopped. FDA also includes a boxed warning about rare reports of persistent dizziness after mefloquine use.
Mefloquine is contraindicated for travelers with a known hypersensitivity to the drug or related compounds (e.g., quinidine, quinine) and in people with active depression, a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia and other major psychiatric disorders, or seizures. Mefloquine should be avoided in people with psychiatric disturbances or a history of depression.
A review of available data suggests that mefloquine can be used safely in people concurrently taking beta-blockers if they have no underlying arrhythmia. Mefloquine is not recommended for people with cardiac conduction abnormalities, however. Any traveler receiving a prescription for mefloquine must also receive a copy of the FDA medication guide [PDF].
Primaquine
Primaquine can cause potentially life-threatening hemolysis in people with glucose-6-phosphate-dehydrogenase (G6PD) deficiency. Rule out G6PD deficiency with a quantitative laboratory test before prescribing primaquine to patients.
Primaquine phosphate has 2 distinct uses for malaria prevention in people with normal G6PD levels: primary prophylaxis in areas with primarily P. vivax, and terminal prophylaxis for travelers who have had prolonged exposure in malaria-endemic areas. Among people with normal G6PD levels taking primaquine, the most common adverse event is gastrointestinal upset; this occurs most commonly if the drug is taken on an empty stomach, and can be minimized or eliminated if it is taken with food.
Primary Prophylaxis
When taken for primary prophylaxis, primaquine should be taken 1–2 days before travel to malaria-endemic areas, daily (at the same time each day) while in the malaria-endemic area, and daily for 7 days after leaving the area (see Table 5-28 for recommended dosages).
Terminal Prophylaxis
In addition to primary prophylaxis, terminal prophylaxis (also known as presumptive antirelapse therapy) generally is indicated for long-term travelers (e.g., military personnel, missionaries, Peace Corps volunteers) with prolonged exposure to P. ovale or P. vivax malaria. Terminal prophylaxis involves taking primaquine toward the end of the exposure period (or immediately thereafter) for the presumptive purpose of eliminating hypnozoites (dormant liver stages) of P. ovale or P. vivax, thereby preventing relapses or delayed-onset clinical presentations of malaria. Because most malaria-endemic areas of the world (except the Caribbean) have ≥1 species of relapsing malaria, travelers to these areas have some risk for acquiring either P. ovale or P. vivax, although the actual risk for an individual traveler is difficult to define.
When indicated, travelers should take primaquine for 14 days after leaving a malaria-endemic area, concurrently with their primary prophylaxis medication. If chloroquine, doxycycline, or mefloquine are used for primary prophylaxis, prescribe primaquine for travelers to take during the last 2 weeks of postexposure prophylaxis. When atovaquone-proguanil is used for primary prophylaxis, travelers can take primaquine during the final 7 days of atovaquone-proguanil, and then for an additional 7 days. If concurrent administration of primary and terminal prophylaxis is not feasible, instruct travelers to take primaquine after completing their primary prophylaxis medication. Primary prophylaxis with primaquine or with tafenoquine (see the following section) obviates the need for terminal prophylaxis.
Tafenoquine
Tafenoquine can cause potentially life-threatening hemolysis in people with G6PD deficiency. Rule out G6PD deficiency with a quantitative laboratory test before prescribing tafenoquine to patients.
Primary Prophylaxis
Tafenoquine (Arakoda 100 mg tablets) can be used to prevent malaria in adults (see Table 5-28 for recommended dosages). Travelers should take a daily loading dose of tafenoquine for 3 days before leaving for a malaria-endemic area; starting 7 days after the loading dose is complete, they should take a weekly maintenance dose while in the malaria-endemic area; then take a final dose in the week after leaving the malaria-endemic area. Doses should be taken on the same day each week.
Tafenoquine is contraindicated in pregnant women and during breastfeeding. Avoid prescribing tafenoquine for people with a history of psychotic disorder; rare psychiatric adverse events have been observed in people with a history of psychotic disorder using higher doses of tafenoquine. The most common adverse events reported with use of tafenoquine are dizziness, gastrointestinal disturbances, headache, and clinically insignificant decreases in hemoglobin. Tafenoquine should be taken with food.
Terminal Prophylaxis
As of 2020, CDC no longer recommends tafenoquine for terminal prophylaxis of P. ovale or P. vivax malaria.
Prophylaxis for Infants, Children & Adolescents
All children traveling to malaria-endemic areas should use recommended prevention measures, which often include taking an antimalarial drug. In the United States, antimalarial drugs are not available in liquid formulation and can taste bitter. Calculate pediatric doses carefully according to the patient’s body weight, but never exceed the adult dose. Pharmacists can pulverize tablets and prepare gelatin capsules for each measured dose. If a child is unable to swallow capsules or tablets, parents should prepare the child’s medication dose by breaking open the gelatin capsule or crushing the pill and mixing the drug with a small amount of something sweet (e.g., condensed milk, chocolate syrup, chocolate spread) to ensure the entire dose is delivered to the child. Giving the dose on a full stomach can minimize stomach upset and vomiting.
Atovaquone-proguanil can be used as prophylaxis for infants and children weighing ≥5 kg (11 lb); prophylactic dosing for children weighing <11 kg (24 lb) constitutes off-label use in the United States. Chloroquine and mefloquine are options for infants and children of all ages and weights, depending on drug resistance at the destination. Doxycycline can be used for children aged ≥8 years. Primaquine can be used for children who are not G6PD-deficient and who are traveling to areas with principally P. vivax. Pediatric dosing regimens are included in Table 5-28.
Prophylaxis During Pregnancy
Malaria infection can be more severe in pregnant than in nonpregnant women. Malaria increases the risk for adverse pregnancy outcomes, including premature birth, spontaneous abortion, and stillbirth; thus, because no prophylaxis regimen is completely effective, advise women who are pregnant or likely to become pregnant to avoid travel to areas with malaria transmission if possible (see Sec. 7, Ch. 1, Pregnant Travelers). If travel to a malaria-endemic area cannot be deferred, an effective prophylaxis regimen and mosquito avoidance measures are essential.
Pregnant women traveling to areas where chloroquine-resistant P. falciparum has not been reported can take chloroquine prophylaxis. Chloroquine has not been found to have harmful effects on the fetus when used in the recommended doses for malaria prophylaxis; therefore, pregnancy is not a contraindication for malaria prophylaxis with chloroquine or hydroxychloroquine.
For travel to areas with known chloroquine-resistant Plasmodium, mefloquine is the only medication recommended for malaria prophylaxis during pregnancy. Studies of mefloquine use during pregnancy have found no indication of adverse effects on the fetus.
Atovaquone-proguanil is not recommended for use during pregnancy because of limited availability of data on its safety, and because other options are available. If other antimalarial drug options are not feasible, however, clinicians and patients should weigh the options, risks, and benefits of using atovaquone-proguanil to make the best decision for the patient. Doxycycline is contraindicated for malaria prophylaxis during pregnancy because of the risk for adverse effects seen with tetracycline, a related drug, on the fetus. These adverse effects include discoloration and dysplasia of the teeth and inhibition of bone growth. Neither primaquine nor tafenoquine should be used during pregnancy; both drugs can be passed transplacentally to a G6PD-deficient fetus and cause hemolytic anemia in utero.
Women planning to become pregnant can use the same medications recommended for use during pregnancy (chloroquine or mefloquine, depending on the area of travel). CDC does not make recommendations about delaying pregnancy after the use of malaria prophylaxis medicines. If the traveler or their health care provider wishes to decrease the amount of antimalarial drug in the body before conception, however, Table 5-29 provides information on the half-lives of the recommended malaria prophylaxis medicines. After 2 half-lives, ≈25% of the drug remains in the body, ≈6% remains after 4 half-lives, and ≈2% remains after 6 half-lives.
Prophylaxis During Breastfeeding
The quantities of antimalarial drugs excreted in the breast milk of lactating women are insufficient to provide adequate protection to nursing infants. Therefore, infants who require prophylaxis should receive the recommended dosages of antimalarial drugs listed in Table 5-28. Because chloroquine and mefloquine can be prescribed safely to infants, infants also can be safely exposed to the small amounts excreted in breast milk. Data about the use of doxycycline in lactating women are very limited; most experts, however, consider the theoretical possibility of adverse events to the infant to be remote.
Although no information is available on the amount of primaquine or tafenoquine that enters human breast milk, test both the woman breastfeeding and the infant for G6PD deficiency before initiating chemoprophylaxis with either one of these drugs. Because data are not yet available on the safety of atovaquone-proguanil prophylaxis in infants weighing <5 kg (11 lb), CDC does not recommend this drug to prevent malaria in women who are breastfeeding infants weighing <5 kg. Atovaquone-proguanil can, however, be used to treat people who are breastfeeding infants of any weight when the potential benefit outweighs the potential risk to the infant (e.g., treating a breastfeeding woman who has acquired P. falciparum malaria in an area of multidrug-resistant strains and who cannot tolerate other treatment options).
Travel to Areas with Chloroquine-Resistant Malaria
Chloroquine-resistant P. falciparum is found in all parts of the world except the Caribbean and countries west of the Panama Canal. Although chloroquine-resistant P. falciparum predominates in Africa, it is found in combination with chloroquine-sensitive P. vivax malaria in South America and Asia. Chloroquine-resistant P. vivax has been confirmed only in Papua New Guinea and Indonesia. For destinations with known chloroquine-resistant Plasmodium spp., in addition to mosquito avoidance measures, prescribe atovaquone-proguanil, doxycycline, mefloquine, or tafenoquine as prophylaxis.
Travel to Areas with Chloroquine-Sensitive Malaria
Areas with chloroquine-sensitive Plasmodium spp. include many Latin American countries where malaria predominantly is caused by P. vivax. Chloroquine-sensitive P. falciparum is present in the Caribbean and Central American countries west of the Panama Canal. For destinations with known chloroquine-sensitive Plasmodium spp., in addition to mosquito avoidance measures, the many effective prophylaxis options include chloroquine, atovaquone-proguanil, doxycycline, mefloquine, and tafenoquine. In countries where P. vivax predominates, primaquine is also an option.
Travel to Areas With Mefloquine-Resistant Malaria
Mefloquine-resistant P. falciparum has been confirmed in Southeast Asia on the borders of Thailand with Burma (Myanmar) and Cambodia, in the western provinces of Cambodia, in the eastern states of Burma on the border between Burma and China, along the borders of Burma and Laos, and in southern Vietnam. For destinations with known mefloquine-resistant Plasmodium spp., in addition to mosquito avoidance measures, prophylaxis options are atovaquone-proguanil, doxycycline, and tafenoquine.
Travel to Areas With Limited Malaria Transmission
For destinations where malaria cases occur sporadically and risk for infection to travelers is considered low, CDC recommends that travelers use mosquito avoidance measures only, and no chemoprophylaxis (see Sec. 2, Ch. 5, Yellow Fever Vaccine & Malaria Prevention Information, by Country).
Changing Medications as a Result of Side Effects During Prophylaxis
Medications recommended for malaria prophylaxis have different modes of action that affect the parasites at different stages of the life cycle. Thus, if the medication needs to be changed because of side effects before a full course has been completed, some special considerations exist (see Table 5-30).
Table 5-30 Malaria chemoprophylaxis: changing medications due to side effects
|
DRUG BEING STOPPED: |
|
|---|---|
DRUG BEING STARTED |
DIRECTIONS FOR USE & COMMENTS |
|
CHLOROQUINE |
Not recommended |
|
DOXYCYCLINE |
Begin taking doxycycline; continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for another 4 weeks after leaving the endemic area. |
|
MEFLOQUINE |
Not recommended |
|
PRIMAQUINE |
This switch would be unlikely because primaquine is recommended as primary prophylaxis for people with normal G6PD activity traveling to areas with mainly Plasmodium vivax. Should that be the case, begin taking primaquine. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for an additional 7 days after leaving the endemic area. |
|
TAFENOQUINE |
Not recommended |
|
DRUG BEING STOPPED: |
|
|---|---|
DRUG BEING STARTED |
DIRECTIONS FOR USE & COMMENTS |
|
ATOVAQUONE-PROGUANIL |
If the switch occurs ≥3 weeks before departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day. Continue taking 1×/day for an additional 7 days after leaving the area. If the switch occurs <3 weeks before departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day, for 4 weeks after the switch. If the switch occurs after departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day, for 4 weeks after leaving the area. |
|
DOXYCYCLINE |
Begin taking doxycycline. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for another 4 weeks after leaving the area. |
|
MEFLOQUINE |
Not recommended |
|
PRIMAQUINE |
Primaquine is recommended as primary prophylaxis for people with normal G6PD activity traveling to areas with mainly P. vivax. Should that be the case, begin taking primaquine. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for an additional 7 days after leaving the area. |
|
TAFENOQUINE |
For people with normal G6PD activity, begin taking tafenoquine as soon as possible after taking the last dose of chloroquine in a malaria-endemic area. Start by taking tafenoquine 1×/day for 3 days, then 1×/week while still in the area. Take 1 final dose during the week after leaving the endemic area. |
|
DRUG BEING STOPPED: |
|
|---|---|
DRUG BEING STARTED |
DIRECTIONS FOR USE & COMMENTS |
|
ATOVAQUONE-PROGUANIL |
If the switch occurs ≥3 weeks before departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day. Continue taking 1×/day for an additional 7 days after leaving the endemic area. If the switch occurs <3 weeks before departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day, for 4 weeks after the switch. If the switch occurs after departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day, for 4 weeks after leaving the area. |
|
CHLOROQUINE |
Not recommended |
|
MEFLOQUINE |
Not recommended |
|
PRIMAQUINE |
This switch would be unlikely because primaquine is recommended as primary prophylaxis for people with normal G6PD activity traveling to areas with mainly P. vivax. Should that be the case, begin taking primaquine. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for an additional 7 days after leaving the endemic area. |
|
TAFENOQUINE |
Not recommended |
|
DRUG BEING STOPPED: |
|
|---|---|
DRUG BEING STARTED |
DIRECTIONS FOR USE & COMMENTS |
|
ATOVAQUONE-PROGUANIL |
If the switch occurs ≥3 weeks before departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day. Continue taking 1×/day for an additional 7 days after leaving the endemic area. If the switch occurs <3 weeks before departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day, for 4 weeks after the switch. If the switch occurs after departure from a malaria-endemic area, take atovaquone-proguanil 1×/day, at the same time each day, for 4 weeks after leaving the area. |
|
CHLOROQUINE |
Not recommended |
|
DOXYCYCLINE |
Begin taking doxycycline. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for another 4 weeks after leaving the endemic area. |
|
PRIMAQUINE |
This switch would be unlikely because primaquine is recommended as primary prophylaxis for people with normal G6PD activity traveling to areas with mainly P. vivax. Should that be the case, begin taking primaquine. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for an additional 7 days after leaving the endemic area. |
|
TAFENOQUINE |
For people with normal G6PD activity, begin taking tafenoquine as soon as possible after taking the last dose of mefloquine in a malaria-endemic area. Start by taking tafenoquine 1×/day for 3 days, then 1×/week while still in the endemic area. Take 1 final dose during the week after leaving the endemic area. |
|
DRUG BEING STOPPED: |
|
|---|---|
DRUG BEING STARTED |
DIRECTIONS FOR USE & COMMENTS |
|
ATOVAQUONE-PROGUANIL |
Begin taking atovaquone-proguanil. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for an additional 7 days after leaving the endemic area. |
|
CHLOROQUINE |
Not recommended |
|
DOXYCYCLINE |
Begin taking doxycycline. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for another 4 weeks after leaving the endemic area. |
|
MEFLOQUINE |
Not recommended |
|
TAFENOQUINE |
Not recommended |
|
DRUG BEING STOPPED: |
|
|---|---|
DRUG BEING STARTED |
DIRECTIONS FOR USE & COMMENTS |
|
ATOVAQUONE-PROGUANIL |
Begin taking atovaquone-proguanil. Continue taking 1x/day, at the same time each day, while in malaria-endemic areas. Take 1x/day for an additional 7 days after leaving the endemic area. |
|
CHLOROQUINE |
Not recommended |
|
DOXYCYCLINE |
Begin taking doxycycline. Continue taking 1×/day, at the same time each day, while in malaria-endemic areas. Take 1×/day for another 4 weeks after leaving the endemic area. |
|
MEFLOQUINE |
Not recommended |
|
PRIMAQUINE |
Not recommended |
Abbreviations: G6PD, glucose-6-phosphate-dehydrogenase
Obtaining Medications Overseas
Medications recommended for malaria prophylaxis might be available at overseas destinations. Combinations of these medications and additional drugs that are not recommended might be commonly prescribed and used in other countries, however. Strongly discourage travelers from obtaining prophylaxis medications while abroad. The quality of these products is not known; products might be produced under substandard manufacturing practices, be counterfeit, contain contaminants, not be protective, or be dangerous. Additional information on medications obtained while traveling can be found in Sec. 6, Ch. 3, . . . perspectives: Avoiding Poorly Regulated Medicines & Medical Products During Travel, and on the FDA website.
Blood Donation After Travel to Malaria-Endemic Areas
People who have been in an area where malaria transmission occurs should defer donating blood after returning from the malaria-endemic area to prevent transmission of malaria through blood transfusion (see Table 5-31).
Risk assessments can differ between travel health providers and blood banks. A travel health provider advising a traveler going to a country with relatively low malaria transmission for a short period of time and engaging in low-risk behaviors might suggest the traveler use only mosquito bite precautions and no prophylaxis. Upon the traveler’s return, however, a blood bank might still choose to defer blood donations from that traveler for 1 year because of travel to an area where transmission occurs.
Table 5-31 US Food and Drug Administration recommendations for deferring blood donation in people returning from malaria-endemic areas
| GROUP |
BLOOD DONATION DEFERRAL |
|---|---|
|
Travelers to malaria-endemic areas |
Not permitted to donate blood for 3 months after travel. |
|
Former residents of malaria-endemic areas |
Not permitted to donate blood for 3 years after departing. If they return to a malaria-endemic area within that 3-year period, they are deferred for an additional 3 years. |
|
People diagnosed with malaria |
Not permitted to donate blood for 3 years after treatment. |
CDC website: Malaria
The following authors contributed to the previous version of this chapter: Kathrine R. Tan, Paul M. Arguin