Volume 13, Number 12—December 2007
Dispatch
Escherichia coli O157:H7 in Feral Swine near Spinach Fields and Cattle, Central California Coast1
Abstract
We investigated involvement of feral swine in contamination of agricultural fields and surface waterways with Escherichia coli O157:H7 after a nationwide outbreak traced to bagged spinach from California. Isolates from feral swine, cattle, surface water, sediment, and soil at 1 ranch were matched to the outbreak strain.
Recent experimental and epidemiologic studies suggest that domestic pigs are biologically competent hosts and a potential reservoir of Escherichia coli O157:H7 (1,2). Cattle are considered the primary reservoir of E. coli O157, but fecal shedding by other domestic livestock and wildlife has been described (3,4). E. coli O157 was isolated from a wild boar in Sweden, but there is limited information on its occurrence in feral swine in the United States (5). We report findings from an environmental and laboratory investigation after a nationwide spinach-associated outbreak of E. coli O157 in which the outbreak strain was isolated from feral swine and other environmental samples.
In September 2006, an outbreak of E. coli O157 was linked to consumption of fresh, bagged, baby spinach, with 26 states and Canada reporting 205 cases of illness and 3 deaths (6). Contaminated product was traced to 1 production date (August 15, 2006) at 1 processing plant and fields located on 4 ranches on the central California coast (7). The outbreak strain was isolated initially from cattle feces collected on September 27, 2006, ≈1 mile from an implicated spinach field on a ranch (ranch A) where numerous free-roaming feral swine were observed. We investigated potential involvement of feral swine in E. coli O157 contamination of spinach fields and surface waterways.
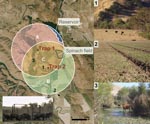
Feral swine were live-captured in traps or hunted and humanely killed during October–November 2006. Two feral swine corral traps were placed 1.4 km apart, and 1.7 km (trap 1) and 1.2 km (trap 2), respectively, from the implicated spinach field (Figure 1). Photographs from digital infrared remote-sensing cameras (Recon Outdoors, Huntsville, AL, USA) were used in combination with sightings and live-capture to ascertain the minimum number of individual feral swine present on the ranch (8). The average population density was calculated on the basis of an estimate of the area sampled by both traps and the estimated mean home range (1.8 km) for feral swine in mainland California by using ArcView version 9.2 (Environmental Systems Research Institute, Redlands, CA, USA) (8).
Colonic fecal samples were collected from 40 feral swine (31 live-captured, 9 hunted); buccal swabs, rectal-anal swabs, and tonsils were analyzed from a subset of 8 animals (Table 1). Additionally, feces from domestic animals (cattle, dog, goat, horse, sheep) and wildlife (bird, coyote, deer, feral swine), surface water and sediment, soil, and well/irrigation water were analyzed. E. coli O157 was cultured by using an extended enrichment–immunomagnetic separation protocol (9,10). PCR analysis was used to confirm the presence of E. coli O157 and virulence factors (9,10). Genotypes of isolates from environmental samples were compared by using 10-loci multilocus variable number tandem repeat analysis (MLVA) and pulsed-field gel electrophoresis (PFGE) after digestion with XbaI and BlnI by using the PulseNet protocol (10–13).
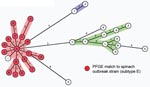
E. coli O157 was cultured from 45 (13.4%) of 335 samples, including cattle and feral swine feces, feral swine colonic feces from necropsy, surface water and sediment, and pasture soil (Table 1). The eaeA, hlyA, and stx2 genes were present in all strains, and the stx1 gene was found in only 1 sample (subtype 5; Table 2, Figure 2). Isolates from 28 environmental samples at ranch A were indistinguishable from the major spinach-related outbreak strain by PFGE (Table 1). In contrast, E. coli O157 isolates from 3 other ranches implicated by traceback did not match the outbreak strain. Molecular typing by MLVA provided higher resolution discrimination between environmental strains (Figure 2). Three major MLVA clusters from ranch A and the surrounding watershed were identified. The cluster containing the outbreak strain (subtype E) is shown in Figure 2, and 16 other highly related subtypes were indistinguishable by PFGE (Table 2).
Ranch A is located in the central coast foothills of San Benito County, where the dominant habitat is coastal oak woodland interspersed with dense riparian vegetation near seasonal waterways (Figure 1). Approximately 2,000 range cattle were grazed on the ranch. Spinach and other leafy green vegetables were grown on a leased portion of the property that was separated from cattle pastures by wire mesh fence. Well water was used for irrigation. No evidence of cattle manure–based fertilizer application, runoff from cattle pastures, or flooding from surface waterways (based on topography) onto the implicated spinach field was found during the investigation (7).
Feral swine were the most abundant wildlife observed on ranch A, and evidence of intrusion, including tracks, rooting, or feces in crop fields and adjacent vineyards, was documented (Figure 1). Birds, black-tailed deer, cottontail rabbits, coyotes, and ground squirrels also were observed, but the population density of these species appeared lower, and their activity was confined mostly to rangeland areas according to visual observations. Swine visited the traps almost continuously from dusk until dawn with peak activity between 5:00
We describe the first, to our knowledge, isolation of E. coli O157 from feral swine in the United States. The percentage of specimens positive for E. coli O157 among feral swine (14.9%) and cattle (33.8%) and the density (4.6 swine/km2) were high compared with results of previous ecologic studies (Table 1) (2–5,8,14,15). Molecular typing of isolates by PFGE and MLVA showed possible dissemination and persistence of the outbreak strain in multiple environmental samples as long as 3 months after the outbreak (Tables 1, 2). MLVA is more reproducible than PFGE and better at discriminating between closely related E. coli O157 isolates (10,12,13). Recovery of related E. coli O157 subtypes by both methods suggested swine-to-swine transmission, interspecies transmission between cattle and swine, or a common source of exposure such as water or soil (Table 2, Figure 2).
Mechanisms of in-field contamination of leafy greens for this and previous outbreaks remain unclear, but hypotheses have emerged. A relatively high density of feral swine near cattle and spinach fields could represent a risk factor for E. coli O157 contamination. Wildlife may be sentinels for E. coli O157 in the produce production environment, or they may be vectors involved in the contamination of plants directly by fecal deposition or indirectly by fecal contamination of surface waterways or soil. Notably, baby spinach is harvested with a lawn mower–like machine that could pick up fecal deposits in the field and thereby contaminate large volumes of product during processing. Fecal loading of surface waterways by livestock and wildlife with subsequent contamination of wells used for irrigation represents another possible route of transmission to plants in the field. Although E. coli O157 was not detected in irrigation water, older agriculture wells at ranch A appeared vulnerable to contamination by surface water (R. Gelting, pers. comm.). Unrecognized environmental and management practices during preharvest and postharvest processing also could have contributed to amplification and dissemination of E. coli O157 in raw spinach.
In summary, E. coli O157 contamination of spinach and other leafy greens is likely a multifactorial process. Additional research is needed to develop and implement effective risk assessment and management practices. For example, studies are needed to determine colonization potential of and levels of fecal shedding by feral swine, and the importance of interspecies transmission to other vertebrate or invertebrate (e.g., flies) populations near agricultural fields.
Dr Jay is a research scientist at the Food and Drug Laboratory Branch at the California Department of Public Health and an affiliate scientist at the Western Institute of Food Safety and Security, University of California, Davis. Her research interests include the molecular epidemiology of zoonotic pathogens and relationships to vertebrate population dynamics and the environment.
Acknowledgments
We thank Richard Geltin, CDC, and the members of the California Food Emergency Response Team, a joint emergency response team of the California Department of Public Health and the US Food and Drug Administration, for their work on the environmental investigation. We also are grateful to the growers, ranchers, and property owners for their cooperation during this study.
Portions of this work were supported by a grant from US Department of Agriculture Cooperative State Research, Education, and Extension Service Section 32.1 (project no. 2006-01240) and Agricultural Research Service projects 5325-42000-044 and -045.
References
- Cornick NA, Helgerson AF. Transmission and infectious dose of Escherichia coli O157:H7 in swine. Appl Environ Microbiol. 2004;70:5331–5. DOIPubMedGoogle Scholar
- Feder I, Wallace FM, Gray JT, Fratamico P, Fedorka-Cray PJ, Pearce RA, Isolation of Escherichia coli O157:H7 from intact colon fecal samples of swine. Emerg Infect Dis. 2003;9:380–3.PubMedGoogle Scholar
- Rice DH, Hancock DD, Besser TE. Faecal culture of wild animals for Escherichia coli O157:H7. Vet Rec. 2003;152:82–3.PubMedGoogle Scholar
- Sargeant JM, Hafer DJ, Gillespie JR, Oberst RD, Flood SJ. Prevalence of Escherichia coli O157:H7 in white-tailed deer sharing rangeland with cattle. J Am Vet Med Assoc. 1999;215:792–4.PubMedGoogle Scholar
- Wahlstrom H, Tysen E, Olsson-Engvall E, Brandstrom B, Eriksson E, Morner T, Survey of Campylobacter species, VTEC O157 and Salmonella species in Swedish wildlife. Vet Rec. 2003;153:74–80.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Ongoing multistate outbreak of Escherichia coli serotype O157:H7 infections associated with consumption of fresh spinach—United States, September 2006. MMWR Morb Mortal Wkly Rep. 2006;55:1045–6.PubMedGoogle Scholar
- California Department of Health Services and US Food and Drug Administration. Investigation of an Escherichia coli O157:H7 outbreak associated with Dole pre-packaged spinach. 2007;1–50 [cited 2007 Apr 17]. Available from http://www.dhs.ca.gov/ps/fdb/html/food/envinvrpt.htm
- Sweitzer RA, Gardner IA, van Vuren D, Boyce WM, Waithman JD. Estimating sizes of wild pig populations in the north and central coast regions of California. J Wildl Manage. 2000;64:531–43. DOIGoogle Scholar
- Himathongkham S, Dodd ML, Yee JK, Lau DK, Bryant RG, Badoiu AS, Optimal enrichment conditions and recirculating immunomagnetic separation (RIMS) for enhanced detection and recovery of low levels of Escherichia coli O157:H7 from fresh leafy produce and surface water. J Food Prot. 2007;70. In press.PubMedGoogle Scholar
- Cooley M, Carychao D, Crawford-Miksza L, Jay MT, Myers C, Rose C, Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS One. 2007;2:e1159. DOIPubMedGoogle Scholar
- Ribot EM, Fair MA, Gautom DN, Cameron SB, Hunter B, Swaminathan B, Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3:59–67. DOIPubMedGoogle Scholar
- Hyytia-Trees E, Smole SC, Fields PA, Swaminathan B, Ribot EM. Second generation subtyping: a proposed PulseNet protocol for multiple-locus variable-number tandem repeat analysis of Shiga toxin-producing Escherichia coli O157 (STEC O157). Foodborne Pathog Dis. 2006;3:118–31. DOIPubMedGoogle Scholar
- Keys C, Kemper S, Keim P. Highly diverse variable number tandem repeat loci in the E. coli O157:H7 and O55:H7 genomes for high-resolution molecular typing. J Appl Microbiol. 2005;98:928–40. DOIPubMedGoogle Scholar
- Atwill ER, Sweitzer RA, Pereira MG, Gardner IA, van Vuren D, Boyce WM. Prevalence of and associated risk factors for shedding Cryptosporidium parvum oocysts and Giardia cysts within feral pig populations in California. Appl Environ Microbiol. 1997;63:3946–9.PubMedGoogle Scholar
- Waithman JD, Sweitzer RA, Van Vuren D, Drew JD, Brinkhaus AJ, Gardner IA, Range expansion, population sizes, and management of wild pigs in California. J Wildl Manage. 1999;63:298–308. DOIGoogle Scholar
Figures
Tables
Cite This Article1This work was presented in part as a poster at the 107th General Meeting of the American Society for Microbiology, Toronto, Ontario, Canada, May 24, 2007.
Table of Contents – Volume 13, Number 12—December 2007
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Michele T. Jay, Food and Drug Laboratory Branch, California Department of Public Health, Western Institute for Food Safety and Security, University of California, 1 Shields Ave, Davis, CA 95616, USA;
Top