Volume 17, Number 9—September 2011
Research
Role of Chlamydia trachomatis in Miscarriage
Abstract
To determine the role of Chlamydia trachomatis in miscarriage, we prospectively collected serum, cervicovaginal swab specimens, and placental samples from 386 women with and without miscarriage. Prevalence of immunoglobulin G against C. trachomatis was higher in the miscarriage group than in the control group (15.2% vs. 7.3%; p = 0.018). Association between C. trachomatis–positive serologic results and miscarriage remained significant after adjustment for age, origin, education, and number of sex partners (odds ratio 2.3, 95% confidence interval 1.1–4.9). C. trachomatis DNA was more frequently amplified from products of conception or placenta from women who had a miscarriage (4%) than from controls (0.7%; p = 0.026). Immunohistochemical analysis confirmed C. trachomatis in placenta from 5 of 7 patients with positive PCR results, whereas results of immunohistochemical analysis were negative in placenta samples from all 8 negative controls tested. Associations between miscarriage and serologic/molecular evidence of C. trachomatis infection support its role in miscarriage.
The incidence of Chlamydia trachomatis infection has dramatically increased during the past 10 years (1). Mostly asymptomatic, untreated C. trachomatis infections are responsible for a large proportion of salpingitis, pelvic inflammatory disease, ectopic pregnancy, and infertility in women. C. trachomatis is a recognized agent of preterm labor and premature rupture of membranes (2,3). However, its role in miscarriage is unclear (2,3).
C. trachomatis has been isolated or detected in cervical smear, urine (4–6), or products of conception (7,8). Nevertheless, none of these studies demonstrated association between isolation of C. trachomatis and miscarriage. However, culturing C. trachomatis is technically difficult, given its strict intracellular life cycle. Even with molecular approaches, detecting C. trachomatis can be difficult because of PCR inhibitors or low number of copies often present in the lesions (4–7). Moreover, infection could be localized at deeper sites not amenable to sampling (9).
Several studies have reported a higher prevalence of C. trachomatis antibodies in spontaneous (10,11) or recurrent (2,9,11,12) miscarriages. The inability to detect immunoglobulin (Ig) M or to isolate C. trachomatis from any of these seropositive patients might suggest that Chlamydia spp. are not directly associated with miscarriage (9,12). Other seroepidemiologic studies have failed to find any correlation between C. trachomatis and spontaneous (13–16) or recurrent miscarriage (17,18).
The main purpose of this study was to investigate whether C. trachomatis is associated with miscarriage. We used molecular, serologic, and immunohistochemical approaches to compare evidence of present and past C. trachomatis infection in women with or without miscarriage.
During November 2006–June 2009, a total of 386 women were prospectively enrolled at the obstetric department of the University Hospital of Lausanne (Lausanne, Switzerland). The miscarriage group comprised 125 women consulting at the emergency gynecology ward for an acute miscarriage. The control group comprised 261 women attending the labor ward with an uneventful pregnancy and without any history of miscarriage, stillbirth, or preterm labor. All women gave written consent, and the local ethical committee approved the study.
We collected demographic and obstetric data prospectively. Placenta (or products of conception in cases of miscarriage), cervicovaginal swab specimens, and serum were sampled at the time of labor and of miscarriage.
All serum samples were tested for IgG and IgA against C. trachomatis with the Ridascreen Chlamydia IgG/IgA Kit (R-biopharm, Darmstadt, Germany) according to the manufacturer’s instructions and by using Dynex DSX (Magellan Biosciences, Chantilly, VA, USA). Cervicovaginal swabs and placenta were extracted by using QIAamp DNA Mini kit (QIAGEN, Hilden, Germany). Samples were screened for C. trachomatis DNA by using a TaqMan real-time PCR specific for the cryptic plasmid of C. trachomatis, as described (19). A PCR inhibition control was used to verify that absence of amplification was not caused by PCR inhibitors. Only 1 of the 386 PCR inhibition controls was negative. This sample was thus retested at a 1:10 dilution.
Hematoxylin and eosin–stained histologic sections of all placentas were investigated for deciduitis, vasculitis, endometritis, or chorioamnionitis. Histologic samples were read blindly by a pedopathologist (M.-C.O.). Samples positive for C. trachomatis by real-time PCR were tested by immunohistochemical analysis (IHC). Presence of C. trachomatis on histologic sections was assessed by using a specific mouse monoclonal antibody, as described (20). To test the placental specimens, we used a commercial Chlamydiaceae family–specific monoclonal antibody directed against the chlamydial lipopolysaccharide (clone AC1-P; Progen, Heidelberg, Germany) at a dilution of 1:200. Detection was performed with the Dako ChemMate detection Kit (Dako, Glostrup, Denmark) according to the manufacturer’s instructions. Antigen retrieval was performed by 10-min enzyme digestion (proteinase K; Dako). Immersion of the slides in peroxidase-blocking solution for 5 min at room temperature resulted in blocking of endogenous peroxidase activity. Specimens were incubated with primary antibody for 1 h. Sections were incubated for 10 min at room temperature with the link-antibody (Dako), followed by 10 min incubation with horseradish peroxidase (Dako) and finally developed in 3-amino, 9-ethyl-carbazole substrate solution for 10 min at room temperature and counterstained with hematoxylin. Using the antibody diluent instead of the primary antibody, we performed a negative control of each section. Moreover, 8 placentas from C. trachomatis PCR-negative patients were randomly selected as negative controls. IHC was blindly read by 2 pathologists with experience in chlamydial IHC (S.B., N.B.) and confirmed by a pedopathologist (M.-C.O.).
We compared demographic data and risk factors of patients with and without miscarriage or C. trachomatis infection by the Pearson χ2 test (or the Fisher exact test when indicated) for categorical variables. For continuous variables, medians were compared by the Wilcoxon-Mann-Whitney test. Multivariate logistic regression was performed to identify factors independently associated with miscarriage or with C. trachomatis infection. Statistical analyses were performed by using Stata version 10.0 (StataCorp LP, College Station, TX, USA).
Of 395 patients, 9 (2.3%) were excluded because of missing serum or vaginal swab samples. Sociodemographic data for the remaining 386 women are shown in Table 1.
A total of 16 (4.2%) patients were positive for IgG and IgA against C. trachomatis, 22 (5.7%) were positive only for IgG against C. trachomatis, and 4 (1.0%) were positive only for IgA against C. trachomatis. Prevalence of IgG against C. trachomatis was higher in the miscarriage group (15.2%) than in the control group (7.3%; p = 0.018) (Table 1). This association between miscarriage and IgG against C. trachomatis remained significant, even after adjustment for age, origin, education, and number of sex partner (odds ratio [OR] 2.3, 95% confidence interval [CI] 1.1–5.1). Similarly, prevalence of IgA against C. trachomatis was higher in the miscarriage group (8.0%) than in the control group (3.8%), but this trend was not significant (p = 0.091) by univariate analysis. When adjusted for age, origin, education, and number of sex partners, the association between miscarriage and IgA against C. trachomatis was significant (OR 2.7, 95% CI 1.1–7.4).
Multivariate logistic regression including all sociodemographic variables (Table 1) and C. trachomatis IgG serologic results identified 5 independent factors positively or negatively associated with miscarriage: C. trachomatis IgG–positive serologic results (OR 2.3, 95% CI 1.1–4.9), age >35 years (OR 2.7, 95% CI 1.6–4.4), European origin (OR 0.3, 95% CI 0.2–0.5), marriage (OR 0.4, 95% CI 0.2–0.7), and 1 lifetime sex partner (OR 0.4, 95% CI 0.2–0.7).
C. trachomatis DNA was more frequently amplified from products of conception or placenta from women with miscarriage (5 [4.0%] women) than from controls (2 [0.7%], p = 0.026). Most patients with a positive PCR result for placenta also had a positive result for vaginal swab specimens (Table 2). Six of the 7 patients with C. trachomatis DNA in the cervicovaginal swab specimen also had positive findings in the placenta. Thus, again, cervicovaginal C. trachomatis DNA was more often detected in patients from the miscarriage group (n = 5, 4.0%) than from the control group (n = 2, 0.7%; p = 0.026). All 7 patients with C. trachomatis DNA in the cervicovaginal swab also exhibited IgG against C. trachomatis, whereas all patients but 1 with C. trachomatis DNA in the placenta exhibited IgG against C. trachomatis (Table 2). Both patients with C. trachomatis DNA and IgG and IgA against C. trachomatis belonged to the miscarriage group.
All placentas were analyzed for inflammation (Figure 1). In the basal plate, inflammatory cells (deciduitis) were present in 15 (39.5%) of 38 patients and 91 (26.1%) of 348 patients with and without C. trachomatis IgG–positive serologic results, respectively (p = 0.081). This trend was observed to a lesser extent when C. trachomatis IgA serologic results were considered (7 [35.0%] of 20 vs. 99 [26.3%] of 376; p = 0.446).
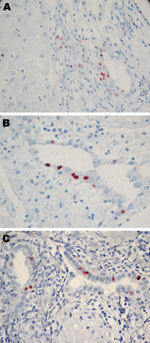
All 8 persons with samples positive for C. trachomatis by real-time PCR in the placenta (n = 7) or cervicovaginal swab specimen (n = 7) were tested by IHC (Table 2; Figure 2). C. trachomatis was confirmed in 4 of 6 placentas from women with miscarriage and in 1 of 2 placentas from women with uneventful pregnancies, whereas none of the 8 C. trachomatis DNA–negative controls randomly selected exhibited the bacteria by IHC. C. trachomatis predominantly localized around endometrial glands of the chorion (Figure 2), associated with different degree of inflammation (Figure 1).
We also compared characteristics of patients with (n = 38) and without (n = 348) C. trachomatis IgG–positive serologic results. Number of pregnancies, parity, marital status, education, number of lifeltime sex partners, and smoking status were all associated with C. trachomatis IgG–positive serologic results by univariate analysis. Women who declined to provide information on the number of sex partners had a C. trachomatis IgG prevalence of 12.2%, whereas none of the 95 women who reported having 1 sex partner had C. trachomatis IgG–positive serologic result. In multivariate analyses, independent factors positively or negatively associated with C. trachomatis IgG–positive serologic results were >2 lifetime sex partners (OR 3.3, 95% CI 1.4–7.7), divorced women (OR 4.9, 95% CI 1.7–14.3), European origin (OR 0.4, 95% CI 0.2–0.9), and attending a university (OR 0.2, 95% CI 0.1–0.6). Age and smoking were not independently associated with C. trachomatis IgG–positive serologic results.
We found an association of spontaneous miscarriage with serologic (p = 0.018) and molecular (p = 0.026) evidence of C. trachomatis infection. Moreover, C. trachomatis in the placenta was documented by specific IHC. C. trachomatis was mainly localized in the epithelial cells of endometrial glands.
Several studies have failed to document an association between C. trachomatis and spontaneous (13–16) or recurrent miscarriage (17,18). However, these studies were conducted >10 years ago, i.e., before the recent dramatic increase in the prevalence and incidence of C. trachomatis infection (1,21,22). Because of improved statistical power, such increased prevalence might indicate an association between C. trachomatis infection and adverse pregnancy outcomes. Second, sensibility and specificity of diagnostic methods have also improved during the past decade. Thus, the high C. trachomatis seroprevalence observed in the control group of several older studies, ranging from 28% to 53% (16,17) was likely to have resulted from a low specificity of the serologic test used at that time. The Chlamydia IgG/IgA kit from R-biopharm we used in the present study exhibited better specificity than did 4 other commercially available tests for detecting IgG against C. trachomatis (23) and is thus more likely to identify a slight but true association. Moreover, the sensitivity of the C. trachomatis TaqMan real-time PCR we used here is high, detecting even <10 DNA copies. This validated assay also detects strains that contain a recently identified 350-bp deletion in the cryptic plasmid (24,25) because the 71-bp DNA fragment amplified is 93 bp downstream from the deletion (19).
The serologic association we observed is unlikely to be due to cross-reactivity with other chlamydial species such as C. abortus (previously classified as C. psittacci senso lato) because we also observed a molecular association with miscarriage. Moreover, the PCR we used was specific at species level because the C. abortus genome contains no cryptic plasmid. Finally, C. abortus has been only infrequently associated with miscarriages in humans (26), mostly after zoonotic exposure.
Miscarriage could be induced by a persistent asymptomatic C. trachomatis infection spreading to the fetal tissue or endometrium. Relatively few miscarriages occur during C. trachomatis primary infection, which explains the absence of association with IgA. That several patients exhibited C. trachomatis–positive serologic results without C. trachomatis DNA suggests that miscarriage might also occasionally be induced by damage from a past chlamydial infection or persistent C. trachomatis antibodies that might interfere with embryonic antigens (2).
A limitation of our study was the absence of investigation of other infectious etiology of miscarriage. Some viruses can produce chronic or recurrent maternal infection. In particular, cytomegalovirus during pregnancy can reach the placenta by hematogenous spread or by ascending route from the cervix. Parvoviruses also have been implicated in the development of repeated fetal loss. Among bacterial infections, Ureaplasma urealyticum, Mycoplasma hominis, and bacterial vaginosis have been mostly associated with miscarriages (27). In addition, several intracellular bacteria such as Coxiella burnettii (28), Brucella abortus (29), and Waddlia chondrophila (11) have been associated with miscarriage.
Our study shows an association between miscarriage and molecular and serologic evidence of C. trachomatis infection. Several previous studies failed to document such an association probably because of the limited number of patients in some of these studies resulting from the lower prevalence of C. trachomatis infection in the late 20th century and to lower sensitivity or specificity of diagnostic methods available at that time. The results of our study suggest that all women experiencing a miscarriage should be screened for C. trachomatis infection and, if positive, adequately treated to prevent recurrent miscarriages. Moreover, preconceptional screening might be proposed to reduce the prevalence of this adverse pregnancy outcome.
Dr Baud is an obstetrician/gynecologist at the University Hospital of Lausanne specializing in maternal/fetal medicine. His research focuses on infectious disease of pregnancy and discovery of emerging pathogens in obstetrics.
Acknowledgments
We thank the midwives and doctors who participated to the sampling of this study. Their involvement was essential to the whole process, and they enthusiastically gave their time to provide information and samples. We thank Francoise Damnon and Andre Baud for computer assistance, Sebastien Aeby for technical help, and Carine Groux and Corinne Grau for C. trachomatis ELISA. We thank Carmen Kaiser and Belinda Senn for performing the chlamydial IHC.
This project was supported mainly by an interdisciplinary grant from the University of Lausanne and partially by grants from the Swiss National Science Foundation (32C0B0-116445 and 310030-130466); by the Institute of Microbiology; and by the Department of Obstetrics and Gynecology. G.G. is supported by the Leenards Foundation through a career award entitled “Bourse Leenards pour la relève académique en médecine clinique à Lausanne.” D.B. is supported by the “Société Académique Vaudoise” through the “Paul Blanc” grant and the SICPA Foundation.
References
- Bébéar C, de Beyrberac B. Genital Chlamydia trachomatis infections. Clin Microbiol Infect. 2009;15:4–10. DOIPubMedGoogle Scholar
- Baud D, Regan L, Greub G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr Opin Infect Dis. 2008;21:70–6. DOIPubMedGoogle Scholar
- Mårdh PA. Influence of infection with Chlamydia trachomatis on pregnancy outcome, infant health and life-long sequelae in infected offspring. Best Pract Res Clin Obstet Gynaecol. 2002;16:847–64. DOIPubMedGoogle Scholar
- Wilkowska-Trojniel M, Zdrodowska-Stefanow B, Ostaszewska-Puchalska I, Redzko S, Przepiesc J, Zdrodowski M. The influence of Chlamydia trachomatis infection on spontaneous abortions. Adv Med Sci. 2009;54:86–90. DOIPubMedGoogle Scholar
- Oakeshott P, Hay P, Hay S, Steinke F, Rink E, Kerry S. Association between bacterial vaginosis or chlamydial infection and miscarriage before 16 weeks’ gestation: prospective community based cohort study. BMJ. 2002;325:1334. DOIPubMedGoogle Scholar
- Sozio J, Ness RB. Chlamydial lower genital tract infection and spontaneous abortion. Infect Dis Obstet Gynecol. 1998;6:8–12.PubMedGoogle Scholar
- Penta M, Lukic A, Conte MP, Chiarini F, Fioriti D, Longhi C, Infectious agents in tissues from spontaneous abortions in the first trimester of pregnancy. New Microbiol. 2003;26:329–37.PubMedGoogle Scholar
- Feist A, Sydler T, Gebbers JJ, Pospischil A, Guscetti F. No association of Chlamydia with abortion. J R Soc Med. 1999;92:237–8.PubMedGoogle Scholar
- Quinn PA, Petric M, Barkin M, Butany J, Derzko C, Gysler M, Prevalence of antibody to Chlamydia trachomatis in spontaneous abortion and infertility. Am J Obstet Gynecol. 1987;156:291–6.PubMedGoogle Scholar
- Vigil P, Tapia A, Zacharias S, Riquelme R, Salgado AM, Varleta J. First-trimester pregnancy loss and active Chlamydia trachomatis infection: correlation and ultrastructural evidence. Andrologia. 2002;34:373–8. DOIPubMedGoogle Scholar
- Baud D, Thomas V, Arafa A, Regan L, Greub G. Waddlia chondrophila, a potential agent of human fetal death. Emerg Infect Dis. 2007;13:1239–43.PubMedGoogle Scholar
- Witkin SS, Ledger WJ. Antibodies to Chlamydia trachomatis in sera of women with recurrent spontaneous abortions. Am J Obstet Gynecol. 1992;167:135–9.PubMedGoogle Scholar
- Grönroos M, Honkonen E, Terho P, Punnonen R. Cervical and serum IgA and serum IgG antibodies to Chlamydia trachomatis and herpes simplex virus in threatened abortion: a prospective study. Br J Obstet Gynaecol. 1983;90:167–70. DOIPubMedGoogle Scholar
- Munday PE, Porter R, Falder PF, Carder JM, Holliman R, Lewis BV, Spontaneous abortion—an infectious aetiology? Br J Obstet Gynaecol. 1984;91:1177–80. DOIPubMedGoogle Scholar
- Coste J, Job-Spira N, Fernandez H. Risk factors for spontaneous abortion: a case-control study in France. Hum Reprod. 1991;6:1332–7.PubMedGoogle Scholar
- Osser S, Persson K. Chlamydial antibodies in women who suffer miscarriage. Br J Obstet Gynaecol. 1996;103:137–41. DOIPubMedGoogle Scholar
- Paukku M, Tulppala M, Puolakkainen M, Anttila T, Paavonen J. Lack of association between serum antibodies to Chlamydia trachomatis and a history of recurrent pregnancy loss. Fertil Steril. 1999;72:427–30. DOIPubMedGoogle Scholar
- Rae R, Smith IW, Liston WA, Kilpatrick DC. Chlamydial serologic studies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;170:782–5.PubMedGoogle Scholar
- Jaton K, Bille J, Greub G. A novel real-time PCR to detect Chlamydia trachomatis in first-void urine or genital swabs. J Med Microbiol. 2006;55:1667–74. DOIPubMedGoogle Scholar
- Borel N, Casson N, Entenza JM, Kaiser C, Pospischil A, Greub G. Tissue microarray and immunohistochemistry as tools for evaluation of antibodies against Chlamydia-like bacteria. J Med Microbiol. 2009;58:863–6. DOIPubMedGoogle Scholar
- Fine D, Dicker L, Mosure D, Berman S. Increasing Chlamydia positivity in women screened in family planning clinics: do we know why? Sex Transm Dis. 2008;35:47–52. DOIPubMedGoogle Scholar
- Abraham S, Toutous-Trellu L, Pechère M, Hugonnet S, Liassine N, Yerly S, Increased incidence of sexually transmitted infections in Geneva, Switzerland. Dermatology. 2006;212:41–6. DOIPubMedGoogle Scholar
- Herrmann B. A new genetic variant of Chlamydia trachomatis. Sex Transm Infect. 2007;83:253–4. DOIPubMedGoogle Scholar
- Baud D, Regan L, Greub G. Comparison of five commercial serological tests for the detection of anti–Chlamydia trachomatis antibodies. Eur J Clin Microbiol Infect Dis. 2010;29:669–75. DOIPubMedGoogle Scholar
- Herrmann B, Törner A, Low N, Klint M, Nilsson A, Velicko I, Emergence and spread of Chlamydia trachomatis variant, Sweden. Emerg Infect Dis. 2008;14:1462–5. DOIPubMedGoogle Scholar
- Pospischil A, Thoma R, Hilbe M, Grest P, Gebbers JO. Abortion in woman caused by caprine Chlamydophila abortus (Chlamydia psittaci serovar 1). Swiss Med Wkly. 2002;132:64–6.PubMedGoogle Scholar
- Nigro G, Mazzocco M, Mattia E. Carlo di RG, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011;24:983–9.PubMedGoogle Scholar
- Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A. Q Fever during pregnancy: a cause of poor fetal and maternal outcome. Ann N Y Acad Sci. 2009;1166:79–89. DOIPubMedGoogle Scholar
- Kurdoglu M, Adali E, Kurdoglu Z, Karahocagil MK, Kolusari A, Yildizhan R, Brucellosis in pregnancy: a 6-year clinical analysis. Arch Gynecol Obstet. 2010;281:201–6. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 17, Number 9—September 2011
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Gilbert Greub, Center for Research on Intracellular Bacteria, Institute of Microbiology, University Hospital Center and University of Lausanne, Bugnon 48, 1011 Lausanne, Switzerland
Top