Volume 18, Number 9—September 2012
CME ACTIVITY - Research
Effectiveness and Timing of Vaccination during School Measles Outbreak
Introduction

MEDSCAPE CME
Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/eid; (4) view/print certificate.
Release date: August 21, 2012; Expiration date: August 21, 2013
Learning Objectives
Upon completion of this activity, participants will be able to:
• Analyze the use of diagnostic testing in cases of influenza-like illness
• Evaluate the use of antiviral medications for outpatient cases of influenza-like illness
• Evaluate the use of antiviral medications for inpatient cases of influenza-like illness
• Assess the care of patients with influenza-like illness and lower respiratory tract infections
CME Editor
Carol E. Snarey, MA, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Carol E. Snarey, MA, has disclosed no relevant financial relationships.
CME AUTHOR
Charles P. Vega, MD, Health Sciences Clinical Professor; Residency Director, Department of Family Medicine, University of California, Irvine. Disclosure: Charles P. Vega, MD, has disclosed no relevant financial relationships.
AUTHORS
Disclosures: Vini Vijayan, MD; and Jennie Jing, MS, have disclosed no relevant financial relationships. Kenneth Zangwill, MD, has disclosed the following relevant financial relationships: served as an advisor or consultant for Merck & Co. Inc.; received grants for clinical research from Novartis.
Abstract
Despite high vaccination coverage in most European countries, large community outbreaks of measles do occur, normally clustered around schools and resulting from suboptimal vaccination coverage. To determine whether or when it is worth implementing outbreak-response vaccination campaigns in schools, we used stochastic outbreak models to reproduce a public school outbreak in Germany, where no vaccination campaign was implemented. We assumed 2 scenarios covering the baseline vaccination ratio range (91.3%–94.3%) estimated for that school and computed outbreaks assuming various vaccination delays. In one scenario, reacting (i.e., implementing outbreak-response vaccination campaigns) within 12–24 days avoided large outbreaks and reacting within 50 days reduced outbreak size. In the other scenario, reacting within 6–14 days avoided large outbreaks and reacting within 40 days reduced the outbreak size. These are realistic time frames for implementing school outbreak response vaccination campaigns. High baseline vaccination ratios extended the time needed for effective response.
Measles is a highly contagious disease that causes illness and death in developing and industrialized countries. Measles has an estimated basic reproduction number (R0) range of 12–40 (1–5), meaning that 1 case introduced into a susceptible (naive) population will produce, on average, that number of secondary cases. Worldwide, measles is the main vaccine-preventable cause of death among children; >31 million cases occur every year, and case-fatality rates for industrialized countries are ≈0.1%–0.2% (6). In 2001, the World Health Organization (WHO) and the United Nations published a global strategic plan for measles (7); the aims of the plan are to sustainably reduce deaths from measles and to interrupt measles virus transmission in countries and regions with elimination objectives.
In 2002, the WHO Region of the Americas was declared free from endemic measles transmission, which was achieved by implementing immunization programs with very high vaccination coverage (>95%). This goal has not been achieved in the WHO European region, for which the target year for measles elimination was 2010. Measles is so highly contagious that the average vaccination coverage in Europe (80%–95%) (8) is not high enough to prevent outbreaks among nonvaccinated persons. The new target year for measles elimination in the WHO European region is 2015 (9).
The only means of protection against measles are prior infection or vaccination. Several studies have focused on the effectiveness of mass outbreak-response vaccination campaigns for controlling measles outbreaks in settings where incidence and morbidity and mortality rates are high. Although some studies suggest that mass outbreak-response vaccination campaigns will not stop measles epidemics because of the rapid spread of the disease (10–12), other studies, which used more recent data, show that outbreak-response vaccination campaigns can successfully reduce illness and death (13–17). The current WHO guidelines recommend mass outbreak-response vaccination campaigns when a measles outbreak is confirmed in settings with a goal of reducing deaths from measles (18) and where most measles cases occur in children <5 years of age. There is no recommendation, however, for implementing outbreak-response vaccination campaigns in settings where incidence and morbidity and mortality rates are low, such as in the WHO European region. In these settings, because of the high baseline vaccination ratio (BVR), a shift in age distribution of measles cases toward older nonvaccinated school children is often noted (19). Consequently, outbreaks initially spread within relatively closed populations of children, such as schools or daycare centers, because of the high rate of social contact among nonvaccinated children in these establishments. Therefore, we focused our study on outbreak-response vaccination campaigns that targeted establishments with children where a measles outbreak was occurring in settings with high BVRs.
A delay from detection of an outbreak to implementation of an outbreak-response vaccination campaign to onset of an effective immune response of those vaccinated is inevitable. Because measles is highly contagious, many children might become infected during that delay. Thus, whether vaccinating children against measles during a school outbreak would substantially affect the outcome of a newly forming epidemic is in doubt (20). In this study, we used stochastic models to estimate the expected size of an outbreak in a school, depending on the delay between detection and implementation of complete school outbreak-response vaccination campaign.
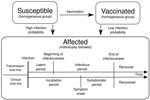
We based our stochastic model design on a combined compartmental and individual-based approach (Figure 1). To set our baseline models, we calibrated the model parameters to fit the data from a real school measles outbreak during which no outbreak-response vaccination campaign was implemented. Although detection of an outbreak depends on notification and surveillance policies, we defined the beginning of the outbreak as the day on which the index case-patient showed clear signs of the disease because that is an objective starting point for the outbreak. We also defined the vaccination delay as the interval between the beginning of the outbreak and the day that the vaccination campaign was implemented at the school. For simplicity, we assumed that all nonvaccinated students were vaccinated on the day of implementation of the campaign. We used the calibrated model parameter values (Table) and investigated the outbreak size distribution by varying the vaccination delay. We computed 100,000 simulation runs for each model with different vaccination delays, ranging from day 0 (the same day that the outbreak began) to 150 days, in intervals of 2 days; day 0 meant that the school outbreak-response vaccination campaign was implemented at the beginning of the outbreak.
The Model
We used a compartmental approach to describe the part of the population not yet infected. We considered 2 subgroups—susceptible and vaccinated—to represent the nonvaccinated and vaccinated school populations, respectively, at the beginning of the outbreak. Susceptible persons were assumed to have been completely unexposed (naive) to measles virus and to have a high probability of becoming infected (Pinf) if they contacted an infectious person. Vaccinated persons were assumed to have acquired protection from measles virus by vaccination and to have a reduced probability of becoming infected (Pinf,vac>0) to account for an imperfect vaccine.
For susceptible (or eventually a vaccinated) persons who became infected, the model took an individual-based approach and followed each person from the time of infection until the end of the simulation. Persons who had had measles before the outbreak were included as affected but were already immune to infection from the beginning of the simulation. Each affected person had individual transmission and clinical time lines, depending on time since infection, which varied from one person to another.
Disease History Time Lines
From the moment a person became infected, and therefore affected, 2 parallel time lines were updated in 1-day steps. The time lines describe the disease history: transmission and clinical (Figure 1).
The transmission time line describes when the affected person became infectious, after a latent period of duration (Dlat), and when that person ceased to be infectious because the person recovered after the infectious period of duration (Dinf) had passed. Durations of these periods were generated randomly from their respective probability distributions (Table). After an affected person recovered, that person was unable become infected again because of acquired life-long immunity. The transmission time line describes the events that dictate the dynamics of the epidemic, but in practice these events are difficult to observe.
In contrast, the clinical time line describes events that can actually be observed. This time line indicates the moment of the rash onset, after the incubation period (Dinc), and the moment when the affected person recovered from the disease, after the symptomatic period (Dsymp) had passed. The values of these periods for each affected person are drawn from the probability distributions indicated in the Table. For simplicity, we assumed that the symptomatic period ended at the same time that the infectious period ended.
Persons who had been infected before the outbreak were considered recovered at the start of our simulations. That is, they were at the end of their disease time lines and did not contribute to the final size of the outbreak.
Infection
We assumed that every person in the school mixed with everybody, i.e., homogeneous mixing, and considered daily contact distribution as shown in the Table. Therefore, each day the number of contacts that an infectious affected person had (ncont) was drawn from this distribution. From these contacts, the number of newly infected susceptible and vaccinated persons was computed according to the infection probabilities, Pinf and Pinf,vac, respectively.
Vaccination
The percentage of vaccinated persons at the beginning of the outbreak was determined by the BVR, and we assumed that vaccination-acquired protection does not wane with time. The high effectiveness of the measles vaccine (5,26) implies that outbreaks occur mostly among those who are not vaccinated. The intervention strategy consisted of vaccinating all school children without documented measles vaccination; the goal was 100% vaccination coverage. This strategy influences only the susceptible persons by reducing their probability of becoming infected to Pinf,vac = (1-VES)Pinf, where VES is the vaccine effectiveness value (Table). Susceptible persons are not protected at the moment of vaccination because the immune response to the vaccine takes time to develop. These persons remain susceptible for a period ([Dimm]; probability distribution is indicated in the Table) after the vaccination day and then become part of the vaccinated group.
Isolation
Usually, persons with measles stay home while recovering from the disease. Therefore, we assumed that after disease symptoms developed (after the incubation period Dinc), infected persons stopped attending school. This absenteeism prevents further contact at school and further spread of the disease, even if the affected person remains infectious at home. For this study, we ignored the possibility of siblings attending the same school.
Model Calibration
We calibrated our models by using data from a retrospective cohort study conducted at a public day school in Duisburg, Germany, where a large measles outbreak had occurred in 2006 (19,27). The first cluster in this epidemic had occurred at that school, providing a typical scenario for the early behavior of an outbreak. The outbreak data consisted of a population of 1,250 schoolchildren 10–21 years of age (median 14 years), of which a high proportion (91.3%–94.3%), were vaccinated; 62 students had a history of measles before the outbreak, and a total of 55 cases were confirmed (5,27). Because no outbreak-response vaccination campaign had been implemented, we calibrated the infection probability (Pinf) in our models to describe this situation. Assuming a BVR of 91.3%, calculated by Wichmann et al. (27), we adjusted the infection probability (Pinf) in our models so that the size distribution of large outbreaks from our simulations peaked at 55 cases. The calibrated Pinf value in combination with our assumed contact distribution (Table) translated into a basic reproduction number of R0≈16, which is consistent with reported basic reproduction number estimations for measles (1–4). The calibrated Pinf value in combination with a BVR of 91.3% yielded roughly an effective reproduction number (Reff) as follows: Reff≈R0[1−(BVR/100%)] = 1.4. In theory, if BVR is high enough that Reff is <1, then 1 case generates on average <1 secondary case, leading to herd immunity effects and no large outbreaks (1–4,28).
In a later study, van Boven et al. (5) used a Bayesian method to estimate the BVR at 94.3%. Considering this BVR, a higher infection probability (Pinf) is needed in our models to reproduce the observed outbreak size, which translates to a higher basic reproduction number, R0≈31, needed to produce an outbreak in a population with such a high BVR. The conditions in this scenario yield an Reff of ≈1.8, indicating that our simulated outbreaks spread more quickly and had higher attack rates than in the less contagious case of R0≈16 and Ref≈1.4. To obtain equivalent outbreak sizes, the attack rate (percentage of affected susceptible persons) has to be higher in a population in which BVR is larger because the number of susceptible children is smaller.
Figure 2 shows the final size distribution histograms for R0≈16 and R0≈31. The typical bimodal distribution predicted by stochastic models appears in both scenarios (28) but is most pronounced for the case of R0≈31. The bimodal distribution arises because it is always possible that chance events will cause a new outbreak to die out before becoming large. We interpreted the local minimum in both distributions of Figure 2 (i.e., an outbreak size of 20) as a natural but arbitrary limit to distinguish between small and large outbreaks. Therefore, although both distributions show a most probable large outbreak size of 55 persons, in the R0≈16 model, 39% of the simulated outbreaks instances become large outbreaks, and in the R0≈31 model, 64% of the instances become large.
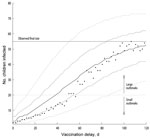
Figure 3 shows the final size distribution of outbreaks as a function of the model vaccination delay indicated in the x-axis for the R0≈16 model. Because of the stochastic nature of the model, as a result of chance events, some outbreaks died out before the intervention was implemented, as could happen in real life. To study the effect of the intervention, we considered only those outbreaks that were still developing at the moment of vaccination. The longer it took to implement the vaccination campaign, the more the outbreak size distributions shifted toward larger outbreaks. Outbreaks were expected to remain small (<20 infected children) if the vaccination delay was 12–24 days. Although the reaction must be quick to avoid an outbreak involving >20 children, a reaction as late as 50 days reduced the final size of large outbreaks in 95% of the simulations. With vaccination delays of >80–90 days, the expected outbreak size was similar to that when no vaccination campaign was implemented.
We also considered the scenario described by van Boven et al. (5), who based the R0≈31 estimate on the same school outbreak with a high BVR (94.3%). The high R0 value of 31 implies that the infection is more contagious than that in our R0≈16 model, leading to a higher effective reproduction number. Figure 4 shows that the outbreak size distributions shifted toward larger outbreaks if the vaccination delay was longer, similar to the R0≈16 model. However, because of the higher infectiousness of the disease, outbreaks spread more quickly than in the models with R0≈16. This provided only a small time frame of 6–14 days to implement the vaccination campaign if the outbreak size was to be kept small (<20 persons). In 95% of the simulations, a vaccination campaign implemented as late as 40 days after start of the outbreak reduced the final size of the outbreak. There was almost no difference between implementing an outbreak-response campaign after ≈60 days and not implementing one at all.
From those outbreaks that did not die out before the vaccination campaign was implemented, we computed the percentage of those that would become large. This percentage can be interpreted as the probability that an outbreak will become large. The dependency of this percentage on the vaccination delay is shown in Figure 5, where a range limited by the larger and smaller R0 cases is considered. The large outbreak percentage was reduced to 31%–59% if the outbreak-response vaccination campaign was implemented with a delay of 28 days, and it was reduced further if the campaign was implemented earlier: 17%–42% with a 21-day delay, 7%–23% with a 14-day delay, and 1%–7% with a 7-day delay. Although the percentage of large outbreaks became increasingly larger as delay to vaccination became larger, later implementation of outbreak-response vaccination campaigns might still substantially reduce the size of large outbreaks (Figure 3 and Figure 4).
To extend our study to different settings, we ran simulations in the same school setting and assumed various BVRs, the lowest being 80%, comparable to the current situation in Europe (8) (Figure 6). Outbreaks with a higher R0 developed more quickly under the same initial conditions and did not become large if the BVR was high enough to achieve herd immunity (>93.8% and >96.8% for R0≈16 and R0≈31, respectively). For lower BVRs, the final size was larger and outbreaks spread more quickly because of a larger effective reproduction number. Therefore, the time frame to react is smaller; e.g., if BVR = 80% and R0≈16, a 1-week delay is already long enough to expect large outbreaks, but a reaction within 3–4 weeks might substantially reduce the outbreak size. Some outbreak sizes were larger than the number of nonvaccinated children in the population, which is explained by imperfect vaccine producing an effect similar to a lower BVR.
We considered a school of 1,250 children, but there are many smaller institutions. In a simulation for a school of 500 children, while conserving the proportions of children with a history of measles and BVR, the boundary separating large and small outbreaks changed to 13 persons. However, results regarding the timing of vaccination remained approximately the same.
We calculated results for 2 reproduction number values, 31 and 16, which belong to a range that is high when compared with published estimates of the basic reproduction number of measles (R0≈8–18) (1–4). This comparison indicates that our results are rather conservative with regard to how quickly the intervention should be implemented. However, it must be noted that our results apply to schools in Europe for which BVRs were average (>80%) before the outbreak (8).
When we considered institutions with smaller populations than that considered in our study, timing for vaccination remained roughly the same. This finding is explained by the association between the time to react effectively and the generation interval of infection, which is the average time it will take for a newly infected person to infect someone else. The generation interval will depend mostly on the within-host disease development, as long as children have enough contacts to transmit the disease, even in schools with ≈300 children (25).
We considered our contact structure to be homogeneous mixing. More intricate contact networks, such as those considering clustering in the different classrooms of the school, popularity of some children, and household contacts with siblings attending the same school, might better resemble reality. Some network structures, contact rates, and superspreading events can influence the speed and growth of an epidemic (29). However, our baseline simulations had peak incidence during weeks 6–11 (16th and 84th percentiles, respectively), mode 7 weeks, for the R0≈31 scenario; and weeks 6–14, mode 8 weeks, for the R0≈16 scenario. These data are consistent with the real outbreak described by Wichmann et al. (27), indicating that the rate of disease spread in our simulations was similar to that in the real outbreak.
At the moment of vaccination, a person might already be infected but not yet symptomatic or might become infected soon after vaccination, before immunity has had time to develop. We assume that in these cases the disease progresses in the same way it does in nonvaccinated persons. However, there is evidence that vaccinating during the incubation period might mitigate the symptoms of measles infection (30). The vaccine might act as postexposure prophylaxis, potentially reducing the infectiousness of the vaccinated persons.
In many schools in Europe a BVR >80% can be found, but in communities with rather low BVRs (e.g., because of religious or philosophic beliefs), measles virus spreads much quicker. For example, in an outbreak originating in an anthroposophic community in Austria during 2008, with a BVR of 0.6%, of the 123 cases in the anthroposophic school of that community, 96% occurred during the first 4 weeks of the outbreak (31), indicating that most cases were either second or third generation (the index case is considered the first generation). An outbreak-response vaccination campaign during such a rapidly spreading outbreak would be effective in a school setting only if implemented within 1 average generation interval (≈9 days) to avoid third-generation infection, which is logistically difficult and requires 100% compliance. However, because school outbreaks are often part of larger community outbreaks, vaccination activities focused on susceptible persons should be promoted in the broader community at any time to prevent further spread of the disease, independent from the time of a school outbreak.
In our study, we assumed 100% compliance to the vaccination strategy. But high compliance to a vaccination campaign cannot be expected in regions with low BVRs associated with religious or philosophical beliefs that are opposed to vaccination. The compliance needed for an intervention to be effective should ensure herd immunity (≈95% vaccinated children). Other complementary measures can be implemented to control measles outbreaks at schools when no vaccination compliance is expected. For example, a measure such as the temporary exclusion of students who lack documented vaccination or whose parents do not agree to vaccination of their children might have a limited effect on preventing further spread of measles by itself (20,32). However, a combination of timely vaccination of all susceptible students at school who agree to be vaccinated and temporary exclusion of those who do not would be the most effective way to control a measles outbreak in a school.
In conclusion, we computed the possible outcomes of a measles outbreak in a school according to the vaccination delay, assuming that all potentially susceptible children in the school were vaccinated on the same day. We found that it is possible to reduce the number of cases during a measles outbreak in a school by applying a schoolwide vaccination strategy within a realistic time frame. Subsequently, because disease tends to spread in schools during the early stages of a city outbreak, reducing the effects of school outbreaks should help reduce the extent of the outbreak in the larger community. We also showed that BVRs that are high (>80%) but not high enough to achieve herd immunity (≈95%) keep the number of susceptible persons low, reduce the size of an outbreak, and reduce the speed at which the disease spreads, thereby increasing the time frame for mounting an effective intervention.
Dr Bonačić Marinović works as a researcher at the Centre for Infectious Disease Control Netherlands, National Institute for Public Health and the Environment (RIVM), Bilthoven, and the Julius Centre for Health Sciences & Primary Care, University Medical Centre Utrecht. His main research interests involve modeling infectious diseases.
Acknowledgment
We thank Peter Teunis, Michiel van Boven, Susan Hahné, and Jacco Wallinga for useful discussions and guidance, and we thank all local and state public health authority staff and Robert Koch Institute staff who were involved in the outbreak investigation and collected the relevant data.
References
- Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature. 1985;318:323–9. DOIPubMedGoogle Scholar
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. New York: Oxford University Press; 1991. p. 70.
- Mossong J, Muller CP. Estimation of the basic reproduction number of measles during an outbreak in a partially vaccinated population. Epidemiol Infect. 2000;124:273–8. DOIPubMedGoogle Scholar
- Wallinga J, Lévy-Bruhl D, Gay NJ, Wachmann CH. Estimation of measles reproduction ratios and prospects for elimination of measles by vaccination in some western European countries. Epidemiol Infect. 2001;127:281–95. DOIPubMedGoogle Scholar
- van Boven M, Kretzschmar M, Wallinga J, O’Neill PD, Wichmann O, Hahné S. Estimation of measles vaccine efficacy and critical vaccination coverage in a highly vaccinated population. J R Soc Interface. 2010;7:1537–44. DOIPubMedGoogle Scholar
- van den Ent MM, Brown DW, Hoekstra EJ, Christie A, Cochi SL. Measles mortality reduction contributes substantially to reduction of all cause mortality among children less than five years of age, 1990–2008. J Infect Dis. 2011;204(Suppl 1):S18–23. DOIPubMedGoogle Scholar
- World Health Organization. Weekly Epidemiological Record. 2002 Feb 15; 77, 7 [cited 2012 Apr 23]. http://www.who.int/wer/2002/wer7707/en/index.html
- European Center for Disease Prevention and Control. European monthly measles monitoring, March 2012. Surveillance reports; 2012 Mar 19 [cited 2010 Apr 23]. http://ecdc.europa.eu/en/publications/Publications/Forms/ECDC_DispForm.aspx?ID=843&MasterPage=1
- Steffens I, Martin R, Lopalco P. Spotlight on measles 2010: measles elimination in Europe—a new commitment to meet the goal by 2015. Euro Surveill. 2010;15:50. DOIPubMedGoogle Scholar
- Aylward RB, Clements J, Olivé JM. The impact of immunization control activities on measles outbreaks in middle and low income countries. Int J Epidemiol. 1997;26:662–9. DOIPubMedGoogle Scholar
- Grenfell BT, Bjørnstad ON, Kappey J. Travelling waves and spatial hierarchies in measles epidemics. Nature. 2001;414:716–23. DOIPubMedGoogle Scholar
- Broutin H, Mantilla-Beniers NB, Simondon F, Aaby P, Grenfell BT, Guégan J-F, Epidemiological impact of vaccination on the dynamics of two childhood diseases in rural Senegal. Microbes Infect. 2005;7:593–9. DOIPubMedGoogle Scholar
- Grais RF, DE Radiguès X, Dubray C, Fermon F, Guerin PJ. Exploring the time to intervene with a reactive mass vaccination campaign in measles epidemics. Epidemiol Infect. 2006;134:845–9. DOIPubMedGoogle Scholar
- Grais RF, Ferrari MJ, Dubray C, Bjørnstad ON, Grenfell BT, Djibo A, Estimating transmission intensity for a measles epidemic in Niamey, Niger: lessons for intervention. Trans R Soc Trop Med Hyg. 2006;100:867–73. DOIPubMedGoogle Scholar
- Grais RF, Conlan AJK, Ferrari MJ, Djibo A, Le Menach A, Bjørnstad ON, Time is of the essence: exploring a measles outbreak response vaccination in Niamey, Niger. J R Soc Interface. 2008;5:67–74. DOIPubMedGoogle Scholar
- Alberti KP, King LA, Burny M-E, Ilunga BK, Grais RF. Reactive vaccination as an effective tool for measles outbreak control in measles mortality reduction settings, Democratic Republic of Congo, 2005–2006. In Health. 2010;2:65–8. DOIGoogle Scholar
- World Health Organizaion. Response to measles outbreaks in measles mortality reduction settings. Immunization, Vaccines and Biologicals; 2009 [cited 2012 Apr 23]. http://www.who.int/immunization/documents/diseases/measles_who_ivb_09_03/en/index.html
- Wichmann O, Siedler A, Sagebiel D, Hellenbrand W, Santibanez S, Mankertz A, Further efforts needed to achieve measles elimination in Germany: results of an outbreak investigation. Bull World Health Organ. 2009;87:108–15. DOIPubMedGoogle Scholar
- Bätzing-Feigenbaum J, Pruckner U, Beyer A, Sinn G, Dinter A, Mankertz A, Spotlight on measles 2010: preliminary report of an ongoing measles outbreak in a subpopulation with low vaccination coverage in Berlin, Germany, January–March 2010. Euro Surveill. 2010;15:13:pii:19527.
- Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001;20:380–91. DOIPubMedGoogle Scholar
- Heymann DL. Control of communicable diseases manual. Washington (DC): American Public Health Association; 2008. p. 349.
- LCI Measles, October 2010. In: Steenbergen JE van, Timen A, Beaujean DJMA, editor. LCI-guidelines infectious disease control edition 2011. Bilthoven (the Netherlands): LCI, Coordinator Infectious Disease Netherlands, 2011. P. 382–392.
- Helfand RF, Kebede S, Gary HE Jr, Beyene H, Bellini WJ. Timing of development of measles-specific immunoglobulin M and G after primary measles vaccination. Clin Diagn Lab Immunol. 1999;6:178–80.PubMedGoogle Scholar
- Mikolajczyk RT, Akmatov MK, Rastin S, Kretzschmar M. Social contacts of school children and the transmission of respiratory-spread pathogens. Epidemiol Infect. 2008;136:813–22. DOIPubMedGoogle Scholar
- Uzicanin A, Zimmerman L. Field effectiveness of live attenuated measles-containing vaccines: a review of published literature. J Infect Dis. 2011;204(Suppl 1):S133–48. DOIPubMedGoogle Scholar
- Wichmann O, Hellenbrand W, Sagebiel D, Santibanez S, Ahlemeyer G, Vogt G, Large measles outbreak at a German public school, 2006. Pediatr Infect Dis J. 2007;26:782–6. DOIPubMedGoogle Scholar
- Bailey NTJ. The total size of a general stochastic epidemic. Biometrika. 1953;40:177–185. doi: http://dx.doi.org/10.1093/biomet/40.1-2.177
- Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–9. DOIPubMedGoogle Scholar
- Sakuta H, Sawada S, Kuroki Y. Severity of measles among patients with incidental postexposure vaccination. Jpn J Infect Dis. 2008;61:304–6.PubMedGoogle Scholar
- Schmid D, Holzmann H, Schwarz K, Kasper S, Kuo H-W, Aberle SW, Measles outbreak linked to a minority group in Austria, 2008. Epidemiol Infect. 2010;138:415–25. DOIPubMedGoogle Scholar
- Wadl M, Siedler A, Krämer W, Haindl ME, Gebrande S, Krenn-Lanzl I, Measles transmission from an anthroposophic community to the general population, Germany 2008. BMC Public Health. 2011;11:474. DOIPubMedGoogle Scholar
Figures
Table
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title: Evaluation of Diagnostic and Therapeutic Approaches for Suspected Influenza A(H1N1)pdm09 Infection, 2009–2010
CME Questions
1. You are seeing a 17-year-old young woman with a history of malaise, low-grade fever, sore throat, and frontal headache for 2 days. You suspect that this patient might have influenza. Based on the results of the current study, what should you consider regarding influenza testing?
A. 99% of patients with a positive test for influenza met CDC-defined criteria for influenza-like illness (ILI)
B. Children had higher rates of positive testing as outpatients compared with adults
C. All tested patients had some indication suggesting influenza infection
D. The rates of positive testing exceeded 50% among both children and adults in the outpatient setting
2. You decide to initiate treatment with oseltamivir as an outpatient. What does the current study demonstrate regarding the use of oseltamivir in the outpatient setting?
A. Adults were more likely than children to receive oseltamivir
B. Nearly half of patients with a positive rapid influenza diagnostic test (RIDT) were not treated according to CDC guidelines
C. Half of patients receiving oseltamivir had a negative RIDT
D. One quarter of patients received oseltamivir within 48 hours of the onset of symptoms
3. The patient returns to your clinic 2 days later with worsening cough, dyspnea, and fever. You decide to admit her to the hospital. What does the current study demonstrate regarding the management of influenza in the inpatient setting?
A. Over 90% of inpatients received oseltamivir
B. Over 90% of inpatients who received oseltamivir had positive influenza testing
C. Most patients prescribed oseltamivir received an incorrect dose or duration of therapy
D. The most common error in prescribing oseltamivir was extending the duration of therapy
4. What else should you consider regarding the management of influenza in the setting of lower respiratory tract infection (LRTI) in the current study?
A. All patients with cases of LRTI were admitted to the hospital
B. Most patients presented within 2 days of symptom onset
C. Two thirds of patients failed to receive appropriate treatment
D. The principal reason that patients failed to receive oseltamivir was the belief of physicians that the drug would not work
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
Related Links
Table of Contents – Volume 18, Number 9—September 2012
| EID Search Options |
|---|
|
|
|
|
|
|




Please use the form below to submit correspondence to the authors or contact them at the following address:
Axel Antonio Bonačić Marinović, Center for Infectious Disease Control, National Institute for Public Health and the Environment (RIVM), PO Box 1, 3720 BA Bilthoven, the Netherlands
Top