Volume 19, Number 5—May 2013
Letter
Scalp Eschar and Neck Lymphadenopathy Caused by Rickettsia massiliae
To the Editor: Scalp eschar and neck lymphadenopathy is a common clinical entity that most frequently affects women and children during spring and fall. It is usually caused by Rickettsia slovaca and R. raoultii. Typical clinical signs are a scalp lesion at the tick bite site and regional, often painful, lymphadenopathy. Acute disease can be followed by residual alopecia at the bite site (1,2). Two designations have been proposed for this syndrome: tick-borne lymphadenopathy and Dermacentor-borne necrosis-erythema-lymphadenopathy (both have been associated with R. slovaca); however, the most generic and all-inclusive term is scalp eschar and neck lymphadenopathy.
R. massiliae belongs to the spotted fever group rickettsiae, is distributed worldwide, and is transmitted by ticks of the genus Rhipicephalus (3). To our knowledge, only 3 cases of R. massiliae infection in humans have been documented and confirmed by molecular methods. The first case was detected in a blood sample from a patient in Italy who had Mediterranean spotted fever (4); the second case was in a patient in southern France who had spotted fever and acute loss of vision (5); and the third case was in a woman in Argentina who had fever, a palpable purpuric rash, and tache noire (3). We report a case of R. massiliae infection that resulted in scalp eschar and neck lymphadenopathy.
On May, 10, 2012, a 13-year-old boy was examined for headache, high fever, and right painful neck and occipital swelling. Six days earlier, a tick had been removed from the top of his scalp, after which signs and symptoms arose and gradually worsened.
Physical examination revealed temperature 39.5°C, pulse rate 70 beats/min, and respiratory rate 20 breaths/min. The boy appeared to be in good condition. An ≈1-cm black eschar was noted at the site of the tick bite. Palpation of the neck revealed painful bilateral adenopathies. Other lymph nodes in the occipital region were enlarged. No exanthema was noted, the liver was palpable 1 cm under the costal margins, and the spleen was not enlarged. Laboratory evaluation indicated blood cell counts and liver and kidney function within reference limits, mild elevation of inflammatory markers (C-reactive protein 1.2 mg/dL [reference <0.5 mg/dL]), and elevated erythrocyte sedimentation rate (43 mm/h). Ultrasonography of the neck confirmed the presence of numerous, enlarged, oval lymph nodes (maximum 17 mm) with hilar vascularity within normal limits. A scalp eschar biopsy sample and acute- and convalescent-phase (day 30) serum samples were sent to the Istituto Zooprofilattico Sperimentale della Sicilia.
The patient was given doxycycline at 100 mg 2 times per day. Signs and symptoms began to improve 48–72 h later and gradually disappeared. Fever was gone after 3 days, and the other symptoms had regressed after 7 days.
Serologic testing for R. conorii was performed by microimmunofluorescence with the R. conorii/R. typhi IgG MIF Kit (Fuller Laboratories, Fullerton, CA, USA). Total DNA was extracted from blood and the eschar by GenElute Mammalian Genomic DNA Miniprep (Sigma-Aldrich, St. Louis, MO, USA). To detect Rickettsia spp. DNA, we tested nucleic acids by PCR with a set of primers that amplify a 256-bp region of the gene encoding the 17-kDa antigen (6). To obtain information about Rickettsia spp., we amplified regions of the genes gltA (7,8), ompA (7), and ompB (9). PCR products were purified by the Wizard SV Gel and PCR Clean-up System (Promega, Madison, WI, USA), quantified, and sent for sequencing to Macrogen Inc. (Amsterdam, the Netherlands).
Obtained sequences were aligned and analyzed by using Bioedit software (Ibis Biosciences, Carlsbad, CA, USA) and ClustalW version 2.0.10 (www.ebi.ac.uk/clustalw). DAMBE (http://dambe.bio.uottawa.ca/dambe.asp) and MEGA (www.megasoftware.net) software were used to obtain similarity percentages among analyzed sequences. To characterize Rickettsia spp., we used nucleotide sequence identity to reference strains (10).
Convalescent-phase serum was positive for R. conorii; IgG titer was 64. Sequence analysis of purified PCR products obtained from the eschar identified the isolate as R. massiliae. With respect to the reference strain R. massiliae, pairwise nucleotide sequence identity was 99% for the gltA gene (GenBank accession no. JN043507), 99% for the ompA gene (accession no. JQ480842), and 97% for the ompB gene (accession no. AF123714). Phylogenetic analysis (Technical Appendix) also confirmed the identity of the Rickettsia species.
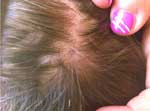
Considering the diagnosis of R. massiliae infection and the patient who had acute vision loss (5), this patient was called back for a fundus examination, which showed no changes. At the time of this visit, a small area of alopecia at the eschar site was observed (Figure). Unfortunately, the tick had been discarded and was not available for genus and species identification.
The presence of R. massiliae in Italy demonstrates that this Rickettsia species can cause scalp eschar and neck lymphadenopathy. Further studies are needed to complete the list of microorganisms that can cause this condition and to understand if they can be associated with minor findings (e.g., alopecia, painful eschar, high fever).
References
- Angelakis E, Pulcini C, Waton J, Imbert P, Socolovschi C, Edouard S, Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after tick bite. Clin Infect Dis. 2010;50:549–51. DOIPubMedGoogle Scholar
- Parola P, Rovery C, Rolain JM, Brouqui P, Davoust B, Raoult D. Rickettsia slovaca and R. raoultii in tick-borne rickettsioses. Emerg Infect Dis. 2009;15:1105–8. DOIPubMedGoogle Scholar
- García-García JC, Portillo A, Nunez MJ, Santibanez S, Castro B, Oteo JA. A patient from Argentina infected with Rickettsia massiliae. Am J Trop Med Hyg. 2010;82:691–2. DOIPubMedGoogle Scholar
- Vitale G, Mansuelo S, Rolain JM, Raoult D. Rickettsia massiliae human isolation. Emerg Infect Dis. 2006;12:174–5. DOIPubMedGoogle Scholar
- Parola P, Socolovschi C, Jeanjean L, Bitam I, Fournier PE, Sotto A, Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl Trop Dis. 2008;2:e338. DOIPubMedGoogle Scholar
- Tzianabos T, Anderson BE, McDade JE. Detection of Rickettsia rickettsii DNA in clinical specimens by using polymerase chain reaction technology. J Clin Microbiol. 1989;27:2866–8 .PubMedGoogle Scholar
- Oteo JA, Portillo A, Santibanez S, Blanco JR, Perez-Martinez L, Ibarra V. Cluster of cases of human Rickettsia felis infection from southern Europe (Spain) diagnosed by PCR. J Clin Microbiol. 2006;44:2669–71. DOIPubMedGoogle Scholar
- Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–89 .PubMedGoogle Scholar
- Choi YJ, Jang WJ, Ryu JS, Lee SH, Park KH, Paik HS, Spotted fever group and typhus group rickettsioses in humans, South Korea. Emerg Infect Dis. 2005;11:237–44 and. DOIPubMedGoogle Scholar
- Zhu Y, Fournier PE, Eremeeva M, Raoult D. Proposal to create subspecies of Rickettsia conorii based on multi-locus sequence typing and an emended description of Rickettsia conorii. BMC Microbiol. 2005;5:11. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 19, Number 5—May 2013
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Antonio Cascio, Programma di Infettivologia Speciale, Medicina Tropicale e delle Migrazioni e Parassitologia, Policlinico “G. Martino,” Via Consolare Valeria n. 1, 98125 Messina, Italia
Top