Volume 19, Number 7—July 2013
Letter
Spotted Fever Group Rickettsiae in Questing Ticks, Central Spain
To the Editor: The number of spotted fever group (SFG) rickettsiae that cause diseases in humans is rapidly increasing (1,2); infections have been described in ticks and humans in Spain (3,4). However, in Castilla-La Mancha, central Spain, where recreational parks and hunting estates are abundant and humans may be exposed to infected ticks, information on such infections is not available. Therefore, it is worthwhile to characterize Rickettsia spp. found in this area for epidemiologic studies and proper diagnosis of possible rickettsial diseases.
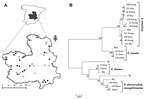
In this study, we obtained 148 questing adult ticks, representing the most abundant species in the area: 12 Dermacentor marginatus, 26 Rhipicephalus bursa, 41 Rh. sanguineus, 15 Rh. turanicus, 8 Rh. pusillus, 2 Haemaphysalis punctata, 11 Hyalomma lusitanicum, and 33 Hyalomma marginatum (5). The ticks were collected from the vegetation at natural sites surveyed in Castilla-La Mancha by blanket dragging with a cotton flannelette during fall 2009 and spring–summer 2010 (Figure, panel A) and classified (5).
Total DNA was extracted from dissected tick internal organs by using the DNeasy Blood & Tissue Kit (QIAGEN, Düsseldorf, Germany) and used to analyze Rickettsia spp. DNA by PCR, cloning, and sequence analysis of the amplicons. At least 3 clones were sequenced for each amplicon. Genes targeted by PCR included fragments of adenosine triphosphate synthase α subunit (atpA), heat-shock protein 70 (dnaK), outer membrane protein A (ompA), outer membrane protein B (ompB), citrate synthase (gltA), 16S rRNA, recA, and initiator protein of DNA replication (dnaA) (6,7). To characterize Rickettsia spp., we compared nucleotide sequence identity to reference strains and carried out multilocus analysis using ompA-ompB sequences and in silico PstI and RsaI restriction analysis of ompA sequences (7).
Ticks were first screened by 16S rRNA PCR, and positive samples were analyzed for all targeted genes. The results showed that 27 (18.2%) of the 148 ticks analyzed were positive for Rickettsia spp. Of these, 11 were confirmed as R. massiliae in Rh. sanguineus, Rh. turanicus, and Rh. pusillus, 3 as R. raoultii in D. marginatus, 2 as R. slovaca in D. marginatus, and 2 as R. sibirica subsp. mongolitimonae in H. marginatum and Rh. pusillus (Figure, panel B). These species had >99% pairwise nucleotide sequence identity to reference strains R. massiliae MTU5 (GenBank accession no. NC_009900), R. slovaca 13-B (accession no. NC_016639), and R. sibirica subsp. mongolitimonae HA-91 (accession no. AHZB00000000) genome sequences for all genes analyzed, and the only R. raoultii reported sequences (accession nos. JQ792107, JQ792166, JQ792134, and NR_043755 for ompB, ompA, gltA, and 16S rRNA, respectively). The sequences obtained in this study were deposited in the GenBank under accession nos. KC427998–KC428040.
Multilocus sequence analysis of ompA-ompB sequences (Figure, panel B) and in silico PstI and RsaI restriction analysis of ompA sequences also confirmed the identity of the Rickettsia spp. identified in this study. As previously shown (7,8), multilocus analysis with ompA-ompB sequences was highly informative about the phylogenetic relationship between Rickettsia spp. (Figure, panel B), with similar results for maximum likelihood, maximum parsimony, and neighbor-joining methods (data not shown). Furthermore, the results suggested the tick vectors for these Rickettsia spp. in the study area (Figure, panel B) match those reported or suspected previously for these Rickettsia spp. (1–4), but for the first time, R. sibirica subsp. mongolitimonae was identified in Hyalomma and Rhipicephalus spp. ticks in Spain (4).
These tick species are frequently found in the same area feeding on Eurasian wild boar (Sus scrofa) and red deer (Cervus elaphus), which may act as hosts for these pathogens (5,9). To test this hypothesis, we determined the seroprevalence for SFG rickettsiae in these host species in Castilla-La Mancha. Serum samples from 235 red deer and 206 wild boar were analyzed for the presence of anti-SFG Rickettsia antibodies by ELISA (Spotted Fever Rickettsia IgG EIA Antibody Kit, Fuller Laboratories, Fullerton, CA, USA). The ELISA was adapted to test ungulate serum specimens by substituting antihuman IgG-horseradish by protein G-horseradish peroxidase (Sigma-Aldrich, Madrid, Spain). Specific SFG-Rickettsia antibodies were detected in 146 (70.9%) of 206 wild boar and 174 (74.0%) of 235 red deer, indicating a high seroprevalence in these species and thus the possibility that they can serve as hosts for these pathogens.
These tick species also infest humans, thus posing a risk for transmission of rickettsiae that are pathogenic in humans (1). In fact, Castilla-La Mancha is one of the regions in Spain where a high number of SFG rickettsioses are reported ([10]; http://pagina.jccm.es/sanidad/salud/epidemiologia/3507.pdf).
In conclusion, these results demonstrate that SFG rickettsiae with public health relevance are found in ticks in central Spain as in other regions in Spain. In central Spain, the widespread distribution of tick vectors and possible wildlife hosts, the presence of persons in tick-infested recreational and hunting areas, and the transstadial and transovarial transmission of the pathogen in ticks may favor transmission to humans.
Acknowledgments
We thank M. Durán-Martínez and R. Sobrino for help with tick surveys.
F. R.-F. and I.G.F.M. are supported by a Juan de la Cierva contract from the Spanish Ministry for Economy and Competitiveness. Research supported by POII09-0141-8176 and European Union FP7 ANTIGONE (Anticipating the Global Onset of Novel Epidemics) project number 278976.
References
- Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719 .PubMedGoogle Scholar
- Uchiyama T. Tropism and pathogenicity of rickettsiae. Front. Microbiol. 2012;3:230.
- Márquez FJ. Spotted fever group Rickettsia in ticks from southeastern Spain natural parks. Exp Appl Acarol. 2008;45:185–94 and. DOIPubMedGoogle Scholar
- Aguirrebengoa K, Portillo A, Santibáñez S, Marín JJ, Montejo M, Oteo JA. Human Rickettsia sibirica mongolitimonae infection, Spain. Emerg Infect Dis. 2008;14:528–9 and. DOIPubMedGoogle Scholar
- Ruiz-Fons F, Fernández de Mera IG, Acevedo P, Höfle U, Vicente J, de la Fuente J, Ticks (Acari: Ixodidae) parasitizing Iberian red deer (Cervus elaphus hispanicus) and European wild boar (Sus scrofa) from Spain: geographical and temporal distribution. Vet Parasitol. 2006;140:133–42 and. DOIPubMedGoogle Scholar
- Fernández de Mera IG, Zivkovic Z, Bolaños M, Carranza C, Pérez-Arellano JL, Gutiérrez C, Rickettsia massiliae in the Canary Islands. Emerg Infect Dis. 2009;15:1869–70 and. DOIPubMedGoogle Scholar
- Torina A, Fernández de Mera IG, Alongi A, Mangold AJ, Blanda V, Scarlata F, Rickettsia conorii Indian tick typhus strain and R. slovaca in humans. Sicily. Emerg Infect Dis. 2012;18:1008–10 . DOIPubMedGoogle Scholar
- Zhu Y, Fournier PE, Eremeeva M, Raoult D. Proposal to create subspecies of Rickettsia conorii based on multi-locus sequence typing and an emended description of Rickettsia conorii. BMC Microbiol. 2005;5:11 and. DOIPubMedGoogle Scholar
- de la Fuente J, Naranjo V, Ruiz-Fons F, Vicente J, Estrada-Peña A, Almazán C, Prevalence of tick-borne pathogens in ixodid ticks (Acari: Ixodidae) collected from European wild boar (Sus scrofa) and Iberian red deer (Cervus elaphus hispanicus) in central Spain. Eur J Wildl Res. 2004;50:187–96. DOIGoogle Scholar
- Bartolomé J, Lorente S, Hernández-Pérez N, Martínez-Alfaro E, Marín-Orsa A, Crespo MD. Estudio clínico-epidemiológico de las rickettsiosis del grupo de las fiebres exantemáticas en Albacete. Enferm Infecc Microbiol Clin. 2005;23:194–6 and. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 19, Number 7—July 2013
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
José de la Fuente, Instituto de Investigación en Recursos Cinegéticos (IREC-CSIC-UCLM-JCCM), Ronda de Toledo s/n, 13005 Ciudad Real, Spain
Top