Volume 22, Number 10—October 2016
Synopsis
Population-Level Effects of Human Papillomavirus Vaccination Programs on Infections with Nonvaccine Genotypes
Figure 3
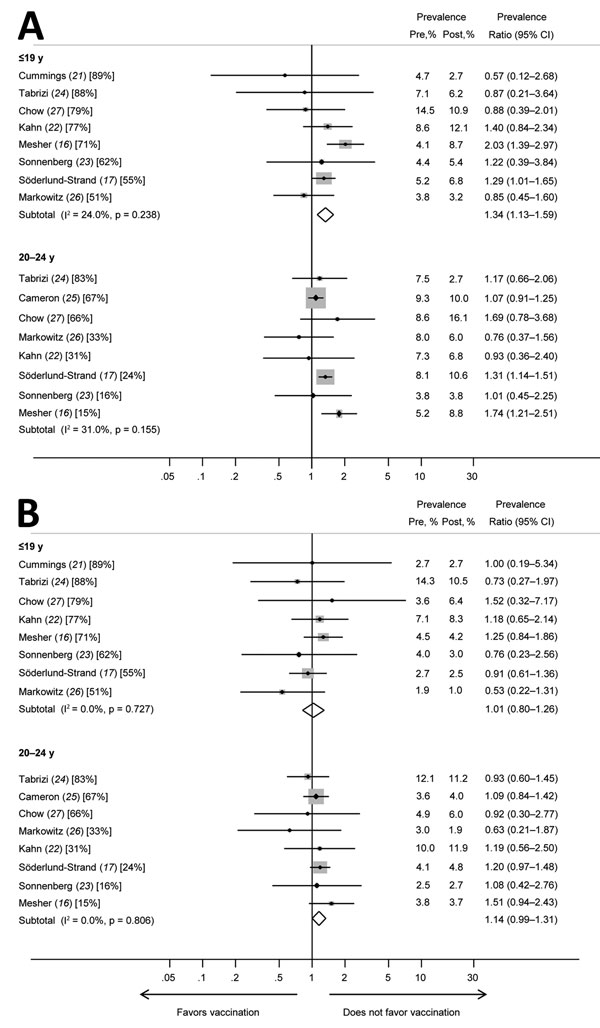
Figure 3. Prevalence ratios and 95% CIs for other high-risk human papillomavirus (HPV) types (HPV52 and HPV58) included in the nonavalent vaccine for girls and women <19 years of age and women 20–24 years of age in studies included in a meta-analysis of changes in prevalences of nonvaccine HPV genotypes after introduction of HPV vaccination. A) HPV52; B) HPV58. Percentages in brackets represent vaccination coverage (>1 dose) for each study and age group. The sizes of the gray boxes around the plot points indicates the relative weight given to each study in the calculation of the summary estimate. The study by Cameron et al. (25) is omitted from analyses for the younger age group because this study included no data for persons <19 years of age. The study by Cummings et al. (21) is omitted from analyses for women 20–24 years of age because the study included no data for this age group. Pre, prevaccination; post, postvaccination.
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9.DOIPubMedGoogle Scholar
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, ; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56.DOIPubMedGoogle Scholar
- Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–35.DOIPubMedGoogle Scholar
- Mesher D, Cuschieri K, Hibbitts S, Jamison J, Sargent A, Pollock KG, Type-specific HPV prevalence in invasive cervical cancer in the UK prior to national HPV immunisation programme: baseline for monitoring the effects of immunisation. J Clin Pathol. 2015;68:135–40.DOIPubMedGoogle Scholar
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–8.DOIPubMedGoogle Scholar
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, ; HPV PATRICIA Study Group. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. DOIPubMedGoogle Scholar
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, ; Broad Spectrum HPV Vaccine Study. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23.DOIPubMedGoogle Scholar
- Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis. 2009;199:926–35.DOIPubMedGoogle Scholar
- Wheeler CM, Castellsagué X, Garland SM, Szarewski A, Paavonen J, Naud P, ; HPV PATRICIA Study Group. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10. DOIPubMedGoogle Scholar
- Cervical Cancer Action. Global maps. Global progress in HPV vaccination. 2014 Sep [cited 2016 Apr 22]. http://www.cervicalcanceraction.org/comments/comments3.php
- Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–80.DOIPubMedGoogle Scholar
- Palmroth J, Merikukka M, Paavonen J, Apter D, Eriksson T, Natunen K, Occurrence of vaccine and non-vaccine human papillomavirus types in adolescent Finnish females 4 years post-vaccination. Int J Cancer. 2012;131:2832–8.DOIPubMedGoogle Scholar
- Malagón T, Drolet M, Boily MC, Franco EL, Jit M, Brisson J, Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:781–9.DOIPubMedGoogle Scholar
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, ; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321–2.DOIPubMedGoogle Scholar
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.DOIPubMedGoogle Scholar
- Mesher D, Panwar K, Thomas SL, Beddows S, Soldan K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: an ongoing cross-sectional study. BMJ Open. 2016;6:e009915.DOIPubMedGoogle Scholar
- Söderlund-Strand A, Uhnoo I, Dillner J. Change in population prevalences of human papillomavirus after initiation of vaccination: the high-throughput HPV monitoring study. Cancer Epidemiol Biomarkers Prev. 2014;23:2757–64.DOIPubMedGoogle Scholar
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.DOIPubMedGoogle Scholar
- Bissett SL, Howell-Jones R, Swift C, De Silva N, Biscornet L, Parry JV, Human papillomavirus genotype detection and viral load in paired genital and urine samples from both females and males. J Med Virol. 2011;83:1744–51.DOIPubMedGoogle Scholar
- Dunne EF, Naleway A, Smith N, Crane B, Weinmann S, Braxton J, Reduction in human papillomavirus vaccine type prevalence among young women screened for cervical cancer in an integrated US healthcare delivery system in 2007 and 2012–2013. J Infect Dis. 2015;212:1970–5.DOIPubMedGoogle Scholar
- Cummings T, Zimet GD, Brown D, Tu W, Yang Z, Fortenberry JD, Reduction of HPV infections through vaccination among at-risk urban adolescents. Vaccine. 2012;30:5496–9.DOIPubMedGoogle Scholar
- Kahn JA, Brown DR, Ding L, Widdice LE, Shew ML, Glynn S, Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130:e249–56.DOIPubMedGoogle Scholar
- Sonnenberg P, Clifton S, Beddows S, Field N, Soldan K, Tanton C, Prevalence, risk factors, and uptake of interventions for sexually transmitted infections in Britain: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet. 2013;382:1795–806.DOIPubMedGoogle Scholar
- Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14:958–66.DOIPubMedGoogle Scholar
- Cameron RL, Kavanagh K, Pan J, Love J, Cuschieri K, Robertson C, Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg Infect Dis. 2016;22:56–64.DOIPubMedGoogle Scholar
- Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137:e20151968.DOIPubMedGoogle Scholar
- Chow EP, Danielewski JA, Fehler G, Tabrizi SN, Law MG, Bradshaw CS, Human papillomavirus in young women with Chlamydia trachomatis infection 7 years after the Australian human papillomavirus vaccination programme: a cross-sectional study. Lancet Infect Dis. 2015;15:1314–23.DOIPubMedGoogle Scholar
- Public Health England. Sexually transmitted infections (STIs): annual data tables. 2015 Jun 23 [cited 2016 Apr 22]. https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables
- Ali H, Donovan B, Wand H, Read TR, Regan DG, Grulich AE, Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032.DOIPubMedGoogle Scholar
- Chow EP, Read TR, Wigan R, Donovan B, Chen MY, Bradshaw CS, Ongoing decline in genital warts among young heterosexuals 7 years after the Australian human papillomavirus (HPV) vaccination programme. Sex Transm Infect. 2015;91:214–9.DOIPubMedGoogle Scholar
- Howell-Jones R, Soldan K, Wetten S, Mesher D, Williams T, Gill ON, Declining genital Warts in young women in england associated with HPV 16/18 vaccination: an ecological study. J Infect Dis. 2013;208:1397–403.DOIPubMedGoogle Scholar
- Lehtinen M, Kaasila M, Pasanen K, Patama T, Palmroth J, Laukkanen P, Seroprevalence atlas of infections with oncogenic and non-oncogenic human papillomaviruses in Finland in the 1980s and 1990s. Int J Cancer. 2006;119:2612–9.DOIPubMedGoogle Scholar
- Laukkanen P, Koskela P, Pukkala E, Dillner J, Läärä E, Knekt P, Time trends in incidence and prevalence of human papillomavirus type 6, 11 and 16 infections in Finland. J Gen Virol. 2003;84:2105–9.DOIPubMedGoogle Scholar
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–99.DOIPubMedGoogle Scholar
- Tota JE, Ramanakumar AV, Villa LL, Richardson H, Burchell AN, Koushik A, Evaluation of human papillomavirus type replacement postvaccination must account for diagnostic artifacts: masking of HPV52 by HPV16 in anogenital specimens. Cancer Epidemiol Biomarkers Prev. 2015;24:286–90.DOIPubMedGoogle Scholar
- Cornall AM, Phillips S, Cummins E, Garland SM, Tabrizi SN. In vitro assessment of the effect of vaccine-targeted human papillomavirus (HPV) depletion on detection of non-vaccine HPV types: implications for post-vaccine surveillance studies. J Virol Methods. 2015;214:10–4.DOIPubMedGoogle Scholar
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 [cited 2016 Apr 22]. http://handbook.cochrane.org/
- Tota JE, Ramanakumar AV, Jiang M, Dillner J, Walter SD, Kaufman JS, Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol. 2013;178:625–34.DOIPubMedGoogle Scholar
- Safaeian M, Rodriguez AC. Invited commentary: multiple human papillomavirus infections and type replacement-anticipating the future after human papillomavirus vaccination. Am J Epidemiol. 2014;180:1076–81.DOIPubMedGoogle Scholar