Volume 22, Number 3—March 2016
Research
Factors Associated with Loss to Follow-up during Treatment for Multidrug-Resistant Tuberculosis, the Philippines, 2012–2014
Figure 1
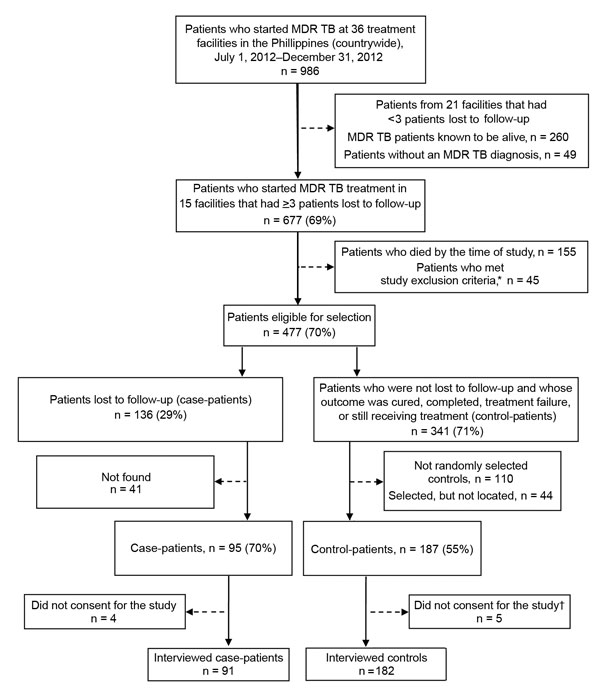
Figure 1. Selection of participants for study of loss to follow-up during treatment for multidrug resistant tuberculosis (MDR TB) in the Philippines, 2012–2014. *Study exclusion criteria were incarceration, age <18 years, enrollment in pharmaceutical clinical trials, and major psychiatric disorder or physical incapacitation. †Control-patients who did not give consent for the study were replaced by other randomly selected eligible patients.
Page created: February 18, 2016
Page updated: February 18, 2016
Page reviewed: February 18, 2016
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.