Volume 15, Number 12—December 2009
Dispatch
Molecular Model of Prion Transmission to Humans
Figure 2
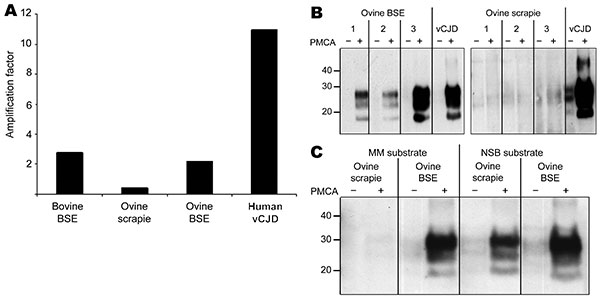
Figure 2. A) Semiquantitative densitometric analysis (optical density × area in mm2) of Western blot data (Figure 1, panel A, top panel), showing the amplification factors (+PMCA/−PMCA) obtained for all 4 seeds (bovine BSE, ovine scrapie, ovine BSE, and human vCJD in the PRNP-129MM substrate. B) Amplification of PrPd associated with ovine BSE (left) and ovine scrapie (right) from each of 3 different sheep in PRNP-129MM substrate as determined by Western blotting using MAb 3F4 to detect PrPres after limited proteinase K digestion. Substrate was seeded with brain homogenates prepared from sheep with confirmed scrapie and BSE such that each PMCA reaction mix contained an equivalent amount of PrPd according to detection of PrPres by Western blot titration after limited proteinase K digestion. PRNP-129MM substrate seeded with vCJD brain homogenate was included as a positive control in each experiment. C) Amplification of PrPd associated with ovine scrapie and BSE in substrates prepared from PRNP-129 methionine homozygous humanized transgenic mouse brain tissue (MM substrate) and NSB substrate. Substrates were prepared as 10% (wt/vol) homogenates in PMCA conversion buffer (10). Each substrate was seeded with brain homogenates prepared from sheep with confirmed scrapie and BSE so that each PMCA reaction mix contained an equivalent amount of PrPd as determined by detection of PrPres by Western blot titration after limited proteinase K digestion. Reaction mixes were divided into 2 lots: 1 was stored immediately at –80°C (−PMCA) and the other was subjected to 48 cycles of PMCA (+PMCA) by using standard conditions (10). After limited proteinase K digestion, PrPres in samples −/+PMCA was detected by Western blotting using MAb 6H4. PMCA, protein misfolding cyclic amplification; BSE, bovine spongiform encephalopathy; vCJD, variant Creutzfeldt-Jakob disease; MM, methionine homozygous; PrPd, disease-associated prion protein; MAb, monoclonal antibody; PrPres, protease-resistant prion protein; NSB, normal ARQ/ARQ sheep brain tissue. Values on the left in panels B and C are in kilodaltons.
References
- Collinge J, Clark AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–6. DOIPubMedGoogle Scholar
- Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, Alperovitch A, Lancet. 1996;347:921–5. DOIPubMedGoogle Scholar
- Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–90. DOIPubMedGoogle Scholar
- Head MW, Bunn TJ, Bishop MT, McLoughlin V, Lowrie S, McKimmie J, Prion protein heterogeneity in sporadic but not variant Creutzfeldt-Jakob disease: UK cases 1991–2002. Ann Neurol. 2004;55:851–9. DOIPubMedGoogle Scholar
- Eloit M, Adjou K, Coulpier M, Fontaine JJ, Hamel R, Lilin T, BSE agent signatures in a goat. Vet Rec. 2005;156:523–4.PubMedGoogle Scholar
- Supattapone S. Prion protein conversion in vitro. J Mol Med. 2004;82:348–56. DOIPubMedGoogle Scholar
- Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–3. DOIPubMedGoogle Scholar
- Jones M, Peden AH, Prowse CV, Groner A, Manson JC, Turner ML, In vitro amplification and detection of variant Creutzfeldt-Jakob disease PrPSc. J Pathol. 2007;213:21–6. DOIPubMedGoogle Scholar
- Yull HM, Ritchie DL, Langeveld JP, van Zijderveld FG, Bruce ME, Ironside JW, Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am J Pathol. 2006;168:151–7. DOIPubMedGoogle Scholar
- Jeffrey M, Martin S, Thomson JR, Dingwall WS, Begara-McGorum I, Gonzalez L. Onset and distribution of tissue prp accumulation in scrapie-affected Suffolk sheep as demonstrated by sequential necropsies and tonsillar biopsies. J Comp Pathol. 2001;125:48–57. DOIPubMedGoogle Scholar
- Gonzalez L, Chianini F, Martin S, Siso S, Gibbard L, Reid HW, Comparative titration of experiment ovine BSE infectivity in sheep and mice. J Gen Virol. 2007;88:714–7. DOIPubMedGoogle Scholar
- Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, Thomson V, Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006;5:393–8. DOIPubMedGoogle Scholar
- Manson JC, Cancellotti E, Hart P, Bishop MT, Barron RM. The transmissible spongiform encephalopathies: emerging and declining epidemics. Biochem Soc Trans. 2006;34:1155–8. DOIPubMedGoogle Scholar