Volume 15, Number 6—June 2009
Synopsis
Diphyllobothriasis Associated with Eating Raw Pacific Salmon
Abstract
The incidence of human infection with the broad tapeworm Diphyllobothrium nihonkaiense has been increasing in urban areas of Japan and in European countries. D. nihonkaiense is morphologically similar to but genetically distinct from D. latum and exploits anadromous wild Pacific salmon as its second intermediate host. Clinical signs in humans include diarrhea and discharge of the strobila, which can be as long as 12 m. The natural life history and the geographic range of the tapeworm remain to be elucidated, but recent studies have indicated that the brown bear in the northern territories of the Pacific coast region is its natural final host. A recent surge of clinical cases highlights a change in the epidemiologic trend of this tapeworm disease from one of rural populations to a disease of urban populations worldwide who eat seafood as part of a healthy diet.
Broad tapeworms such as Diphyllobothrium latum and D. nihonkaiense are exotic parasites that grow as long as 12 meters in the small intestine. By the mid-19th century, infection with the Japanese broad tapeworm was known to be contracted by eating salmon (Figure 1) and was considered to be infection with D. latum until 1986, when Yamane et al. revised the identification of the Japanese broad tapeworm and established the new species D. nihonkaiense (1). Both tapeworms exploit freshwater copepods as their first intermediate host. However, in contrast to D. latum, which uses freshwater fish such as perch, char, and pike as the second intermediate host, D. nihonkaiense uses anadromous fish, Oncorhynchus spp., such as O. masou (masu salmon), O. gorbuscha (pink salmon), and O. keta (chum salmon), which migrate across the northern Pacific Ocean to the Sea of Okhotsk and the Bering Sea (2,3). Recent studies have demonstrated complete mitochondrial genomes of D. nihonkaiense and D. latum (4,5). These genomes have not only rendered species diagnosis more reliable, but they have also provided a wealth of genetic markers that could be useful for investigating their population genetics, ecology, and epidemiology.
Diphyllobothriasis nihonkaiense was once endemic to coastal provinces of central and northern Japan, where salmon fisheries thrived. However, in the past several decades, regions with endemic diphyllobothriasis nihonkaiense have disappeared from Japan, yet the infection has been perpetuated among urban people who eat sushi and sashimi. Although the number of clinical cases of the infection in large cities has fluctuated some in the past 20 years, the incidence was particularly high in 2008. Moreover, clinical cases caused by D. nihonkaiense have been emerging even in European countries (6–9), suggesting that the globalization of this tapeworm disease is probably due to the worldwide expansion of commercial sales of fresh or frozen wild Pacific salmon. We outline the current situation of diphyllobothriasis nihonkaiense in Japan, together with its still-mysterious ecology and life cycle.
We retrospectively examined annual case numbers of diphyllobothriasis nihonkaiense in 2 institutes; the Department of Medical Zoology of the Kyoto Prefectural University of Medicine in Kyoto (MZ) and the Department of Infectious Diseases of the Tokyo Metropolitan Bokutoh Hospital (BH) in Tokyo. MZ is the sole institute specializing in research and diagnosis of parasitic diseases in Kyoto city (population 1.4 million). BH is one of the major public hospitals in metropolitan Tokyo.
From 1988 through 2008, a total of 149 cases of diphyllobothriasis have been recorded: 95 at MZ and 54 at BH. Diphyllobothriasis nihonkaiense was diagnosed by morphologic appearance and taxonomic characteristics of the strobila (body of the mature tapeworm) passed in feces of a person who had a history of eating salmon or a habit of eating sushi or sashimi, which are normally composed of sea fish, often salmon. DNA sequences of the tapeworm cox1 and/or nad3 genes were also analyzed from most (42) patient specimens obtained since 2004; results confirmed the identification of D. nihonkaiense. Molecularly confirmed D. latum, from humans or fish, has not been reported in Japan.
Annual incidence rates of the clinical cases at MZ and BH show an apparent surge in recent years (Figure 2). In a broad assumption that the case numbers at MZ represent all cases of this tapeworm infection in Kyoto, the average incidence in the past 20 years was 0.32 cases per 100,000 population per year, and that in 2008 was 1.0 case per 100,000 population. Incidence throughout Japan has not been estimated because a nationwide investigation has never been conducted. Nevertheless, these case numbers at MZ and BH suggest that D. nihonkaiense infection is equally as prevalent in Japan as D. latum is in some European countries (10).
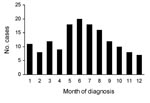
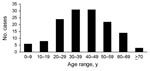
Most patients regularly ate sushi and sashimi. Approximately half could recall that they ate raw or undercooked salmon in the past 6 months. Analyses of 149 cases at MZ and BH showed that the disease occurred during all seasons but that prevalence peaked in early summer (Figure 3). Every age group was affected, from 3 to 77 years. Most patients were 20–59 years of age, which probably reflects more frequent consumption of sushi and sashimi by persons in this age group than in other age groups (Figure 4). Twice as many men than women were affected.
The signs and symptoms caused by D. nihonkaiense differ little from those caused by D. latum. All 149 patients had consulted physicians after passing tapeworm strobila. Average length of the strobila was 83 cm (range 5–400); patients reported that the strobila tore somewhere along its length when they tried to pull it out. The patients also frequently reported abdominal pain or discomfort and several episodes of diarrhea before passing the strobila, but few complained of substantial weight loss. Of the 149 patients, 73 were treated at MZ, BH, or affiliated institutions. Treatment with anthelminthics (praziquantel for most; bithionol, paromomycin, or sodium amidotrizoate and meglumine amidotrizoate [Gastrografin; Bayer Schering Pharma AG, Berlin, Germany] for a few with older cases) showed that 69 (95%) of 73 patients were infected with 1 tapeworm, 2 were infected with 2 tapeworms, and 2 were infected with 3 tapeworms. The tapeworms obtained measured 50–1,200 cm (average 334 cm). The length of the strobila was not associated with the age or sex of the patient, suggesting that all age groups and both sexes are equally susceptible to this tapeworm.
Pernicious (megaloblastic) anemia has been reported in some patients infected with D. latum (11). Among the patients with D. nihonkaiense infection reported here, low hemoglobin concentration (<12 g/dL) was found in 2 of 43 patients examined. Mild eosinophilia (absolute count >600/μL) was also found in 4 of 37 patients examined. A causal relationship between the anemia or eosinophilia and diphyllobothriasis nihonkaiense for these patients was not determined because neither the type of anemia nor the outcome of anemia or eosinophilia after treatment was examined.
Approximately half of the wild Pacific salmon sold in Japan are caught in the coastal areas of northern Japan, and the other half are imported from Far East Russia and the Pacific coast of North America. Salmon-harvesting rivers run through neither Kyoto nor Tokyo. Suzuki et al. (12) investigated plerocercoids (infective larvae) in wild Pacific salmon caught in waters off the coast of northern Japan and sent to Tokyo fish markets during March–July, 2000–2002. They showed that plerocercoids were found in 24 (51%) of 47, 10 (12%) of 82, and 5 (19%) of 27 samples of chum, masu, and pink salmon, respectively. Using PCR-based DNA sequence analysis targeting the cox1 and nad3 genes, they also showed that all plerocercoids recovered were identified as D. nihonkaiense and that 26 chum salmon caught during autumn lacked such infection. This finding implies that wild salmon caught in spring and early summer pose a higher risk for human infection than autumn-caught salmon, consistent with the observation that the incidence of human infection peaks in early summer (Figure 3).
Whether all salmon harvested in the coastal waters off Japan originated from rivers in Japan is unknown. Oshima and Wakai (13) investigated the characteristics of masu salmon harboring diphyllobothriid plerocercoids; rate of infection was 27%. They suggested that these masu salmon probably originated from rivers in Russia despite having been captured in the waters off the coast of Japan and unloaded at Japanese ports. To the contrary, an investigation of mature masu salmon captured in the rivers in Hokkaido showed a plerocercoid infection rate of 20%, although no plerocercoids were found in masu salmon juveniles that stayed in the rivers for 1.5 years before migrating to the sea (14).
Thus, although earlier exhaustive studies have indicated that the first intermediate host of D. nihonkaiense is the freshwater zooplanktonic copepod Cyclops strenuus (15), whether freshwater is the place of transmission of the parasite from the copepod to salmon remains controversial. Some researchers have been examining a hypothesis that Japanese masu salmon are infected with the plerocercoid not in freshwater but in the sea during their migration through the Sea of Okhotsk, possibly through another intermediate host that links the freshwater copepod and the wild salmon at sea (14). So far, no such intermediate host has been discovered.
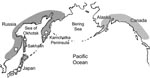
Until recently, diphyllobothriasis nihonkaiense had been reported almost exclusively in Japan. In northern communities bordering the Pacific, several additional diphyllobothriid species—D. klebanovskii, D. ursi, D. latum, D. dendriticum, and D. dalliae—have been implicated in human infections (16–20). In Far East Russia, D. klebanovskii, which also uses wild Pacific salmon as its second intermediate host, is the most common cause of human diphyllobothriasis (16,17). Recent molecular studies of the DNA sequences of the 18S rDNA, internal transcribed region 1, cox1, and nad3, clearly indicated the synonymy of D. klebanovskii to D. nihonkaiense, indicating that D. nihonkaiense is distributed not only in Japan but also in Far East Russia up to the Kamchatka Peninsula and that brown bears are its natural final host (21).
In 1980, on the Pacific coast of the United States, an outbreak of diphyllobothriasis was associated with consumption of Pacific salmon (22), but species identification of the tapeworm was not conducted. More recently, several clinical cases diagnosed by tapeworm DNA sequencing as D. nihonkaiense have emerged in Europe (6–8). These patients had eaten raw Pacific salmon, probably imported from the Pacific coast of North America. Another case, in a tourist to North America who had eaten raw sockeye salmon from British Columbia, was also diagnosed as caused by D. nihonkaiense. (9). These reports suggest a far broader geographic distribution of D. nihonkaiense than previously believed (Figure 5).
However, whether D. nihonkaiense in these regions consists of a biologically homogeneous population is still uncertain. The most enigmatic result of the molecular studies of D. nihonkaiense cox1 and nad3 genes is the presence of 2 deeply divergent lineages that are not defined by the localities of the samples examined so far (21). Thus, further studies are needed to look for an association between the host species and/or geographic localities and the 2 genotypes of D. nihonkaiense.
D. nihonkaiense is not the sole tapeworm species carried by wild Pacific salmon. On the Pacific coast of North America, D. ursi has been isolated from brown bears, black bears, and humans (18,19,23,24). The plerocercoid of D. ursi is found predominantly in sockeye salmon (O. nerka) and occasionally in coho salmon (O. kisutch). A major difference between D. ursi and D. nihonkaiense (D. klebanovskii) is their plerocercoid stage: plerocercoids of D. ursi encyst on stomach serosa of salmon (18), and plerocercoids of D. nihonkaiense (D. klebanovskii) have been found mainly in the body musculature of chum, masu, and pink salmon (1–3). In some South American countries, cultivated Atlantic salmon (Salmo salar) have been implicated as the source of D. latum infection (25,26).
The epidemiology of diphyllobothriasis nihonkaiense has changed drastically from rural to urban areas because of the rapid expansion of the transport system for fresh and frozen fish to meet a demand for seafood in healthy diets. The uninterrupted occurrence of diphyllobothriasis nihonkaiense in urban areas implies that the D. nihonkaiense tapeworm perpetuates its natural life cycle successfully between salmon and its final host animals in northern territories of the Pacific Ocean; however, its definite natural life cycle remains to be elucidated. Freezing and storing at –20 °C for 7 days or –35 °C until solid and storing at –35 °C for 15 hours is sufficient to kill parasites, although these conditions may not be suitable for freezing particularly large fish, e.g., those thicker than 6 inches (27).
It seems that the general public in Japan is only vaguely aware of the possible risk for parasitic diseases associated with eating sushi and sashimi made from marine fish. Although some information on this health risk is provided through means such as health education programs open to the public or television programs, the emphasis is generally on the risk for anisakiasis, one of the most prevalent parasitic diseases among Japanese. Persons are generally underinformed, especially about the risk of diphyllobothriasis from eating raw salmon. Moreover, people like sushi and sashimi made of never-frozen fish far better than that made from frozen fish. Consumers and retailers should be made aware of the risk for tapeworm infection posed by eating raw or undercooked wild salmon.
Dr Arizono is a professor of parasitology at Kyoto Prefectural University of Medicine. His research interests focus on the epidemiology and pathogenesis of helminthic diseases.
Acknowledgment
This study was supported in part by grants-in-aid from the Ministry of Health, Labor and Welfare of Japan (H19-Shinko), the Japan Health Sciences Foundation (KHA2031), and Ohyama Health Foundation.
References
- Yamane Y, Kamo H, Bylund G, Wikgren BJ. Diphyllobothrium nihonkaiense sp. nov (Cestoda: Diphyllobothriidae)—revised identification of Japanese broad tapeworm. Shimane J Med Sci. 1986;10:29–48.
- Eguchi S. Studies on Dibothriocephalus latus, with special reference to the second intermediate host in Japan [in German]. Trans Soc Pathol Jpn. 1929;19:567–70.
- Ando K, Ishikura K, Nakakugi T, Shimono Y, Tamai T, Sugawa M, Five cases of Diphyllobothrium nihonkaiense infection with discovery of plerocercoids from an infective source, Oncorhynchus masou ishikawae. J Parasitol. 2001;87:96–100.PubMedGoogle Scholar
- Nakao M, Abmed D, Yamasaki H, Ito A. Mitochondrial genomes of the human broad tapeworms Diphyllobothrium latum and Diphyllobothrium nihonkaiense (Cestoda: Diphyllobothriidae). Parasitol Res. 2007;101:233–6. DOIPubMedGoogle Scholar
- Park JK, Kim KH, Kang S, Jeon HK, Kim JH, Littlewood DT, Characterization of the mitochondrial genome of Diphyllobothrium latum (Cestoda: Pseudophyllidea)—implications for the phylogeny of eucestodes. Parasitology. 2007;134:749–59. DOIPubMedGoogle Scholar
- Yera H, Estran C, Delaunay P, Gari-Toussaint M, Dupouy-Camet J, Marty P. Putative Diphyllobothrium nihonkaiense acquired from a Pacific salmon (Oncorhynchus keta) eaten in France; genomic identification and case report. Parasitol Int. 2006;55:45–9. DOIPubMedGoogle Scholar
- Wicht B, de Marval F, Peduzzi R. Diphyllobothrium nihonkaiense (Yamane et al., 1986) in Switzerland: first molecular evidence and case reports. Parasitol Int. 2007;56:195–9. DOIPubMedGoogle Scholar
- Shimizu H, Kawakatsu H, Shimizu T, Yamada M, Tegoshi T, Uchikawa R, Diphyllobothriasis nihonkaiense: possibly acquired in Switzerland from imported Pacific salmon. Intern Med. 2008;47:1359–62. DOIPubMedGoogle Scholar
- Wicht B, Scholz T, Peduzzi R, Kuchta R. First record of human infection with the tapeworm Diphyllobothrium nihonkaiense in North America. Am J Trop Med Hyg. 2008;78:235–8.PubMedGoogle Scholar
- Dupouy-Camet J, Peduzzi R. Current situation of human diphyllobothriasis in Europe. Euro Surveill. 2004;9:31–5.PubMedGoogle Scholar
- Pathogenesis of the tapeworm anaemia. BMJ. 1976;2:1028.
- Suzuki J, Murata R, Yanagawa Y, Araki J. Identification of Diphyllobothrium nihonkaiense by PCR-based approach [in Japanese]. Clinical Parasitology. 2006;17:22–4.
- Oshima T, Wakai R. Epidemiology of Diphyllobothrium latum infection in Japan, with special reference to infection of cherry salmon [in Japanese]. Jpn J Antibiot. 1983;36:566–72.PubMedGoogle Scholar
- Awakura T, Sakaguchi S, Hara T. Studies on parasites of masu salmon, Oncorhynchus masou—observations on the seasonal occurrence of Diphyllobothrium latum plerocercoid [in Japanese]. Scientific Reports of the Hokkaido Fish Hatchery. 1985;40:57–67 [cited 2009 Apr 1]. Available from http://rms1.agsearch.agropedia.affrc.go.jp/contents/JASI/pdf/PREF/34-0679.pdf
- Eguchi S. Studies on Dibothriocephalus latus, with special reference to its life history in Japan [in Japanese]. Byorigaku Kiyo. 1926;3:1–66.
- Muratov IV, Posokhov PS. Causative agent of human diphyllobothriasis—Diphyllobothrium klebanovskii sp. n. Parazitologiia. 1988;22:165–70.PubMedGoogle Scholar
- Muratov IV, Posokhov PS, Romanenko NA, Zimin AS, Glazyrina GF. The epidemiological characteristics of diphyllobothriasis caused by Diphyllobothrium klebanovskii in the Amur River basin [in Russian]. Med Parazitol (Mosk). 1992;3:46–7.PubMedGoogle Scholar
- Rausch R. Studies on the helminth fauna of Alaska. XXI. Taxonomy, morphological variation, and ecology of Diphyllobothrium ursi n. sp. provis. on Kodiak Island. J Parasitol. 1954;40:540–63. DOIPubMedGoogle Scholar
- Rausch RL, Hillard DK. Studies on the helminth fauna of Alaska. XLIX. The occurrence of Diphyllobothrium latum (Linnaeus, 1758) (Cestoda: Diphyllobothriidae) in Alaska, with notes on other species. Can J Zool. 1970;48:1201–19. DOIPubMedGoogle Scholar
- Curtis MA, Bylund G. Diphyllobothriasis: fish tapeworm disease in the circumpolar north. Arctic Med Res. 1991;50:18–24.PubMedGoogle Scholar
- Arizono N, Shedko M, Yamada M, Uchikawa R, Tegoshi T, Takeda K, Mitochondrial DNA divergence in populations of the tapeworm Diphyllobothrium nihonkaiense and its phylogenetic relationship with Diphyllobothrium klebanovskii. Parasitol Int. 2009;58:22–8. DOIPubMedGoogle Scholar
- Ruttenber AJ, Weniger BG, Sorvillo F, Murray RA, Ford SL. Diphyllobothriasis associated with salmon consumption in Pacific Coast states. Am J Trop Med Hyg. 1984;33:455–9.PubMedGoogle Scholar
- Margolis L, Rausch RL, Robertson E. Diphyllobothrium ursi from man in British Columbia—first report of this tapeworm in Canada. Can J Public Health. 1973;64:588–9.PubMedGoogle Scholar
- Frechette JL, Rau ME. Helminths of the black bear in Quebec. J Wildl Dis. 1977;13:432–4 [cited 2009 Apr 1]. Available from http://www.jwildlifedis.org/cgi/reprint/13/4/432.pdf
- Sampaio JL, de Andrade VP, Lucas Mda C, Fung L, Gagliardi SM, Santos SR, Diphyllobothriasis, Brazil. Emerg Infect Dis. 2005;11:1598–600.PubMedGoogle Scholar
- Cabello FC. Salmon aquaculture and transmission of the fish tapeworm. Emerg Infect Dis. 2007;13:169–71.PubMedGoogle Scholar
- Anonymous . Parasites. In: Fish and fisheries products hazards and controls guidance, 3rd ed. Rockville (MD): US Food and Drug Administration; 2001. p. 65–72.
Figures
Cite This ArticleTable of Contents – Volume 15, Number 6—June 2009
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Naoki Arizono, Department of Medical Zoology, Kyoto Prefectural University of Medicine, Kamikyo-ku, Kyoto 602-8566, Japan:
Top