Volume 17, Number 12—December 2011
Research
Candidate Cell Substrates, Vaccine Production, and Transmissible Spongiform Encephalopathies
Figure 2
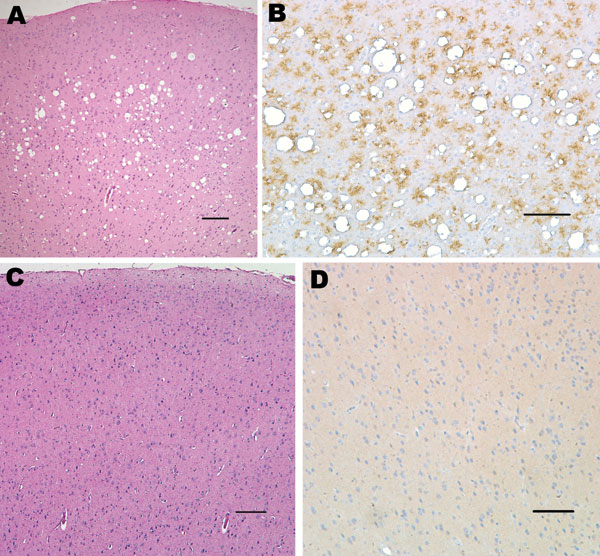
Figure 2. Histopathologic analysis of squirrel monkey inoculated with bovine spongiform encephalopathy agent (A, B). Spongiform degeneration in the cerebral cortex (A), adjacent section showing abundant prion protein (PrP) immunopositivity (B). Squirrel monkey without transmissible spongiform encephalopathy (C, D). Cerebral cortex with no spongiform degeneration (C), absence of PrP positivity in the cerebral cortex (D). Panels A and C correspond to sections stained with hematoxylin and eosin; panels B and D were sections immunostained with PrP antibody 6D11. Scale bars = 150 μm.
Page created: November 30, 2011
Page updated: November 30, 2011
Page reviewed: November 30, 2011
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.