Volume 17, Number 12—December 2011
Research
Lineage and Virulence of Streptococcus suis Serotype 2 Isolates from North America
Abstract
We performed multilocus sequence typing of 64 North American Streptococcus suis serotype 2 porcine isolates. Strains were sequence type (ST) 28 (51%), ST25 (44%), and ST1 (5%). We identified nonrandom associations between STs and expression of the virulence markers suilysin (SLY), muramidase-relased protein (MRP), and extracellular factor (EF). Expression of pili encoded by the srtF and srtG pilus clusters was also nonrandomly associated with STs. ST1 strains were SLY+ EF+ MRP+ srtF pilus+ srtG pilus−. ST25 strains were SLY− EF− MRP− srtF pilus− srtG pilus+, and most ST28 strains were SLY− MRP+ EF− srtF pilus+ srtG pilus+. ST28 isolates proved essentially nonvirulent in a mouse infection model; ST25 strains showed moderate virulence and ST1 isolates were highly virulent. ST1 is responsible for a high proportion of S. suis disease in humans worldwide. Its presence in North America indicates that potential zoonotic S. suis outbreaks in this continent cannot be disregarded.
Streptococcus suis causes meningitis and septicemia in pigs and is a zoonotic agent (1). In the Western hemisphere, human S. suis disease is infrequent and usually affects workers in the swine industry. However, S. suis is the most commonly reported cause of streptococcal meningitis in adults in Vietnam and the second in Thailand (2,3). Two outbreaks of human S. suis disease have occurred in People’s Republic of China, affecting hundreds of persons and causing 39 deaths (4). Most cases of animal and human S. suis infection have been caused by serotype 2 strains (5). The percentage of S. suis serotype 2 strains recovered from diseased pigs and the number of cases of human disease is lower in North America than in other parts of the world (6,7).
Multilocus sequence typing (MLST) has shown that S. suis serotype 2 strains can be divided into at least 16 sequence types (STs). Closely related STs are grouped in the so-called ST complexes. Although ST complexes 1, 27, and 87 dominate the S. suis population, most invasive isolates belong to the ST1 complex (8). For example, most strains isolated from human patients in Japan were ST1 (9), whereas those causing the human outbreaks in People’s Republic of China were ST7, included in the ST1 complex (10,11). However, Takamatsu et al. showed that 80% of the isolates recovered from blood or cerebrospinal fluid of humans in Thailand belonged to STs grouped in the ST27 complex (12).
Most of the S. suis serotype 2 strains genotyped so far by MLST originated in Europe and Asia (8–12). Isolates from Canada and the United States have received less attention. In this study, we used MLST to genotype a relatively large collection of US and Canadian S. suis serotype 2 strains.
S. suis Field Strains
Sixty-four strains of S. suis serotype 2 isolated from pigs with clinical disease in different and nonrelated farms in major swine production areas of Canada and the United States were used. For comparison purposes, 19 porcine and 1 human S. suis serotype 2 strains isolated in Japan and 12 human S. suis serotype 2 strains isolated in Thailand were included (12,13). All strains are listed in Table A1.
MLST and Phylogenetic Analysis
S. suis genomic DNA was prepared from overnight cultures by using the QIAamp DNA Minikit (QIAGEN, Valencia, CA, USA) following the manufacturer’s instructions. MLST was performed by PCR amplification and DNA sequencing of the cpn60, dpr, recA, aroA, thrA, gki, and mutS genes as described (8). For each isolate, the alleles at each of the 7 loci defined the ST. MLST information in the S. suis database (http://ssuis.mlst.net) identified the phylogenetic position of strains. eBURST software (14) was used to identify S. suis clonal complexes and to display the overall structure of the population.
PCRs for Virulence Markers and Pili Cluster Genes
Amplification of sly, mrp, and epf genes was performed by PCR as described (6). Genes in the srtF and srtG pilus clusters were amplified by PCR by using the primers and conditions described by Takamatsu et al. (13).
MRP, EF, and Pili Expression and Hemolysis Assays
S. suis strains were grown in Todd-Hewitt broth at 37°C (at 28°C for detection of the srtG pilus). Bacteria were harvested by centrifugation during the late exponential phase of growth, and supernatants were concentrated 10-fold by using Ultrafree-MC centrifugal filters (Millipore Corp., Bedford, MA, USA). Expression of extracellular factor (EF) and muramidase-released protein (MRP) was determined by Western blotting of the concentrated supernatant fraction by using monoclonal antibodies against MRP or EF, as described (15). Mutanolysin extracts were prepared from pelleted bacteria as described (16,17) and used to detect pili encoded by the srtF and srtG pilus clusters by Western blotting with antibodies directed against the major subunit of each pilus (16,17). The ability of strains to lyse horse erythrocytes (an indication of the production by the strains of the hemolysin known as suilysin, SLY) was determined as described (6).
Experimental Infection of Mice
All animal experiments followed the guidelines of the Canadian Council on Animal Care and were approved by the Ethics Committee, Université de Montréal. We used a validated CD1 mouse infection model (18). In a first experiment, 60 female 6-week-old mice (Charles River Laboratories, Wilmington, MA, USA) were divided in 4 groups. Group 1 was inoculated with ST1 strain P1/7; groups 2 and 3 received ST25 strains 89-1591 and 1085543, respectively. Group 4 received ST28 strain 1088563. The inocula (5 × 107 CFU/animal) were delivered intraperitoneally. Mice were monitored 3×/d for 10 days for clinical signs and assigned clinical scores as described (18). Blood was collected daily from the tail vein (5 μL) and at necropsy by cardiac puncture and used to evaluate bacterial load by plating onto sheep blood agar plates and enumeration. Colonization of the liver and spleen of infected animals was evaluated at necropsy as described (18). A second experiment was performed essentially as described above, but the mice received a 10-fold higher dose of ST28 strains 1088563, 1054471, and 1097205. In this second experiment, groups contained 5 mice.
Most of the 64 strains from North America were ST28 (n = 33) or ST25 (n = 28). Together, these 2 STs accounted for 95% of all S. suis serotype 2 strains from North America that were investigated (Table 1). However, a higher ST28 prevalence was true only for the United States; most strains from Canada were ST25. The remaining 3 strains belonged to ST1, which is commonly found in Europe and Southeast Asia.
Nonrandom Association between STs and Expression of Virulence Markers
SLY (encoded by the sly gene), MRP (mrp gene), and EF (epf gene) are virulence markers that have been used in elaborated genotypic and phenotypic schemes to try to predict the virulence of a given S. suis strain (1,19). For example, Silva et al. designed a multiplex PCR test that can discriminate between at least 6 naturally occurring genetic variants of mrp, named mrps, mrp, mrp*, mrp**, mrp***, and mrp**** (20). We investigated possible associations between STs and these widely used markers in our collection of S. suis serotype 2 strains from North America. To assess whether associations found are independent of the geographic origin of the strains, we included 32 described (12,13) S. suis serotype 2 strains of STs 28, 25, and 1 isolated in Japan and Thailand (Table A1).
Independently of geographic origin, we found clear, nonrandom associations between STs and expression of virulence markers. All but 2 ST1 strains had the phenotype SLY+MRP+ EF+. All ST25 strains were SLY−MRP−EF− and all ST28 strains were SLY−MRP (or its variants)+ EF− (Table 2). Most ST1 strains had an sly+mrp+epf+ genotype, in agreement with results of previous reports (11–13). ST25 and ST28 strains had an sly− genotype and, with the exception of 3 ST28 strains, an epf− genotype. No clear relationships were found between ST25 strains and a particular mrp gene variant genotype. All but 3 ST25 strains were positive by PCR for 1 mrp gene variant, yet none of these strains expressed the protein (Table 2). In a recent report, all mrp+/MRP− strains that were investigated (of various S. suis serotypes) had truncations or point mutations in the mrp gene that prevented expression of MRP (6). Although we have not sequenced the mrp gene in our collection of strains, we hypothesize that similar genetic rearrangements are likely to explain the mrp+/MRP− results we observed in ST25 strains in this study. Three mrp gene variants were associated with ST28, although variant mrp was the most prevalent (85%) among this ST.
Nonrandom Association between STs and Expression of Pili
Takamatsu et al. reported associations between particular STs and the presence or absence of putative pilus gene clusters, designated srtBCD, srtE, srtF, and srtG clusters (13). All ST25 and ST28 strains investigated by these authors were positive by PCR for all genes in the srtF and srtG pilus clusters (13). Consistently, we found that all ST25 and ST28 strains in our collection were positive for all genes in these 2 pilus clusters (Table 3). Furthermore, by using specific antibodies directed against the major pilin subunits (16,17), we identified a clear, nonrandom association between ST28 strains and expression of both pili (Table 3). However, although all ST25 strains expressed the srtG pilus, none produced the srtF pilus (Table 3).
It has been shown that one ST25 isolate from Canada, which does not have a discrete srtF pilus cluster and is unable to express the srtF pilus, is nonetheless PCR positive for each of the individual srtF genes because PCR amplicons can be generated from homologs of these genes found at various genome locations (13,16). We hypothesized that the ST25 strains analyzed in our study have a genetic organization similar to that ST25 isolate. Consistent with our hypothesis, our attempts to amplify the srtF pilus cluster in ST25 strains by using a primer pair annealing to the first and last gene of the srtF cluster were unsuccessful (data not shown). All the ST1 strains had the srtF cluster genes but, with the exception of 3 strains, not the srtG cluster genes. When we assessed the pilus phenotype by Western blotting, all ST1 strains expressed the srtF pilus but none expressed the srtG pilus (Table 3). The reason(s) the 3 ST1 strains that have the srtG cluster genes do not express the corresponding pilus are currently under investigation.
Mouse Infection Model
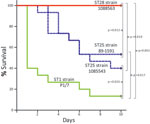
Inasmuch as the MLST data showed that more than half of the strains from North America analyzed were ST28 and the second most represented ST was ST25, we performed a comparison of the virulence of representative ST25 and ST28 strains by using a standardized mouse infection model (18). For comparison, we included the well-characterized and highly virulent ST1 strain P1/7. Most mice in the ST1 group showed severe clinical signs of septicemia, such as depression, swollen eyes, weakness, and prostration during the first 24 hours postinoculation. Several mice died of septicemia during the first 2 days of the trial, and the remaining animals were humanely killed for ethical reasons at day 3 postinoculation (Figure 1). S. suis was isolated in pure cultures at high titers (>1 × 107 CFU/mL) from blood samples and organs, such as the liver and spleen, of septicemic animals in the ST1 group (>1 × 107 CFU/0.5 g of tissue in most animals).
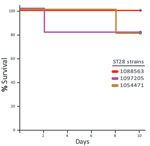
The virulence of ST25 strains was intermediate. They caused moderate clinical signs and relatively low mortality among inoculated mice (Figure 1). Statistical analysis demonstrated that ST25 strains were significantly less virulent than ST1 strains. However, ST25 strains were significantly more virulent than ST28 strains. In fact, no mice in the ST28 group died (Figure 1) or showed clinical signs associated with S. suis infection, with the exception of slight depression immediately after inoculation, which subsided after 24 hours postinoculation. Bacteria could not be isolated from the blood of most mice in this group >48 hours postinoculation, and we could not isolate S. suis from different organs at necropsy (results not shown). Given this surprising absence of clinical signs, we repeated the experiment by inoculating 3 additional groups of 5 mice each with the previously used and 2 other ST28 strains by using an infective dose that was 10-fold higher than the one previously used. Despite this increased infective dose, similar low virulence was observed for ST28 strains (Figure 2).
In this article, we show that most S. suis isolates from North America belong to ST28 and ST25 and that strains of these STs are significantly less virulent than ST1 strains. Although ST28 strains were essentially nonvirulent for mice, ST25 strains were of intermediate virulence and able to induce severe disease.
With a population of ≈115 million pigs, Canada and the United States combined are second only to the People’s Republic of China in terms of swine production. Although S. suis infections are a main cause of postweaned piglet deaths in North America, the prevalence of S. suis serotype 2 strains is much lower on this continent than in other regions of the world (6,7). We show here that in North America the most common STs among S. suis serotype 2 strains are ST28 and ST25. By using a mouse infection model, we also show that S. suis serotype 2 ST28 and ST25 strains are of lower virulence than ST1 strains. In contrast to Europe and Asia, where >60% of virulent serotype 2 isolates are ST1 (21–23), in North America only a small percentage (5%) of strains belonged to this more virulent ST.
Only 3 cases of S. suis serotype 2 in locally infected humans have been reported in North America (5). Our results suggest that this low prevalence of human infections might be connected to the lower virulence of the circulating serotype 2 strains among the swine population in North America. In addition to a low prevalence of ST1 strains, we did not find any strains in our collection from North America belonging to STs 101, 102, 103, and 104, which are agents of human disease in Thailand (12). On the basis of its low frequency of isolation, we speculate that the ST1 strains we identified were introduced in North America by importation of animals. Human travel might also contribute to dissemination of ST1 strains, as exemplified by a reported case of human S. suis meningitis caused by an ST1 strain involving a patient who contracted S. suis in the Philippines but in whom clinical signs appeared only after he returned to the United States (24). The deadly human outbreaks in Asia caused by ST1 complex strains (2–5) and the fact that ST1 strains are replacing at a fast pace STs of lower virulence and causing human disease in countries such as Vietnam and Thailand (21,25,26) highlight that maintaining a low prevalence of ST1 strains among the swine population in North America is crucial for animal and human health. Of note, the only locally acquired human infection in the United States described so far (27) was caused by an ST1 strain (M. Gottschalk, unpub. data).
Another concern for the swine industry and for public health authorities is the presence in North America of S. suis ST25 strains. Many human cases reported in Thailand and 2 cases in Canada of S. suis serotype 2 disease were caused by ST25 strains (5,12). On the other hand, we found that strains of the most prevalent ST28 are of low virulence. Two strains shown here to be ST28 (1330 and 0891; see Table A1) had been reported as nonvirulent S. suis serotype 2 (8). No human S. suis cases attributable to ST28 strains have been reported in North America. However, all ST28 strains included in this study were isolated from diseased pigs, and 1 human case in Japan and 1 human case in Thailand were caused by ST28 strains (9,12). Nonvirulent S. suis strains have been hypothesized to cause disease in immunocompromised animals or humans who have a concurrent infection with another bacterial or viral pathogen(s) (5). Porcine S. suis infections in North America are usually associated with a concomitant infection with the porcine respiratory and reproductive virus (1). We do not know the immunologic status of the animals from which the ST28 strains were isolated to test the aforementioned hypothesis. Toward this goal, however, we are developing a co-infection model of S. suis and porcine respiratory and reproductive virus.
Our results provide evidence that genotyping schemes based on sly, mrp, epf, and pilus cluster genes, although useful in discriminating highly virulent ST1 strains from other groups (8,13,20), are of limited use in differentiating between ST25 and ST28 strains. Although not ideal because protein expression levels may be affected by many factors, typing methods based on protein expression of these markers might a priori differentiate these STs of different virulence. The fact that ST25 strains do not express MRP or the srtF pili, yet they are more virulent than ST28 strains, further demonstrates the dispensability of these factors for the full virulence of S. suis (16,28). Our results also highlight that subunit vaccines based on purified MRP or srtF pilus subunits might be of little use to counter S. suis infections caused by ST25 strains.
Our work provides more support to the longstanding hypothesis that S. suis serotype 2 strains in North America are of lower virulence than strains from Eurasia. However, we do not yet understand the reasons for this lower virulence. The genome sequences of several S. suis serotype 2 ST1 and an ST25 strains have been published or made available (25,29,30). Genome sequencing of a larger number of additional S. suis strains of these and other STs could help elucidate the genetic basis of virulence differences among strains of this swine pathogen and zoonotic agent.
Dr Fittipaldi is a postdoctoral fellow in the Center for Molecular and Translational Human Infectious Diseases Research of the Methodist Hospital Research Institute in Houston, Texas, USA. His primary research interest is the molecular basis of streptococcal pathogen–host interactions.
Acknowledgment
This study was supported by grants from the Natural Sciences and Engineering Research Council of Canada to M.G. (no. 154280 and Discovery Accelerator Supplement 380299) and Minister of Economic Development, Innovation and Export Trade, China-Quebec Collaboration grants to M.G and J.X. (no. 2008FA31830 and 2008DFA31830, respectively). N.F. is partially supported by the Canadian Institutes of Health Research.
References
- Higgins R, Gottschalk M. Streptococcocal diseases. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of swine. Ames (IA): Blackwell Publishing; 2006. p. 769–83.
- Mai NTH, Hoa NT, Nga TV, Linh LD, Chau TT, Sinh DX, Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis. 2008;46:659–67. DOIPubMedGoogle Scholar
- Suankratay C, Intalapaporn P, Nunthapisud P, Arunyingmongkol K, Wilde H. Streptococcus suis meningitis in Thailand. Southeast Asian J Trop Med Public Health. 2004;35:868–76.PubMedGoogle Scholar
- Yu H, Jing H, Chen Z, Zheng H, Zhu X, Wang H, Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:914–20.PubMedGoogle Scholar
- Gottschalk M, Segura M, Xu J. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim Health Res Rev. 2007;8:29–45. DOIPubMedGoogle Scholar
- Fittipaldi N, Fuller TE, Teel JF, Wilson TL, Wolfram TJ, Lowery DE, Serotype distribution and production of muramidase-released protein, extracellular factor and suilysin by field strains of Streptococcus suis isolated in the United States. Vet Microbiol. 2009;139:310–7. DOIPubMedGoogle Scholar
- Messier S, Lacouture S, Gottschalk M. Distribution of Streptococcus suis capsular types from 2001 to 2007. Can Vet J. 2008;49:461–2.PubMedGoogle Scholar
- King SJ, Leigh JA, Heath PJ, Luque I, Tarradas C, Dowson CG, Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J Clin Microbiol. 2002;40:3671–80. DOIPubMedGoogle Scholar
- Chang B, Wada A, Ikebe T, Ohnishi M, Mita K, Endo M, Characteristics of Streptococcus suis isolated from patients in Japan. Jpn J Infect Dis. 2006;59:397–9.PubMedGoogle Scholar
- Ye C, Bai X, Zhang J, Jing H, Zheng H, Du H, Spread of Streptococcus suis sequence type 7, China. Emerg Infect Dis. 2008;14:787–91. DOIPubMedGoogle Scholar
- Ye C, Zhu X, Jing H, Du H, Segura M, Zheng H, Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg Infect Dis. 2006;12:1203–8. DOIPubMedGoogle Scholar
- Takamatsu D, Wongsawan K, Osaki M, Nishino H, Ishiji T, Tharavichitkul P, Streptococcus suis in humans, Thailand. Emerg Infect Dis. 2008;14:181–3. DOIPubMedGoogle Scholar
- Takamatsu D, Nishino H, Ishiji T, Ishii J, Osaki M, Fittipaldi N, Genetic organization and preferential distribution of putative pilus gene clusters in Streptococcus suis. Vet Microbiol. 2009;138:132–9. DOIPubMedGoogle Scholar
- Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186:1518–30. DOIPubMedGoogle Scholar
- Vecht U, Wisselink HJ, van Dijk JE, Smith HE. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect Immun. 1992;60:550–6.PubMedGoogle Scholar
- Fittipaldi N, Takamatsu D, de la Cruz Domínguez-Punaro M, Lecours MP, Montpetit D, Osaki M, Mutations in the gene encoding the ancillary pilin subunit of the Streptococcus suis srtF cluster result in pili formed by the major subunit only. PLoS ONE. 2010;5:e8426. DOIPubMedGoogle Scholar
- Okura M, Osaki M, Fittipaldi N, Gottschalk M, Sekizaki T, Takamatsu D. The minor pilin subunit Sgp2 is necessary for assembly of the pilus encoded by the srtG cluster of Streptococcus suis. J Bacteriol. 2011;193:822–31. DOIPubMedGoogle Scholar
- Domínguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J Immunol. 2007;179:1842–54.PubMedGoogle Scholar
- Baums CG, Valentin-Weigand P. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim Health Res Rev. 2009;10:65–83. DOIPubMedGoogle Scholar
- Silva LM, Baums CG, Rehm T, Wisselink HJ, Goethe R, Valentin-Weigand P. Virulence-associated gene profiling of Streptococcus suis isolates by PCR. Vet Microbiol. 2006;115:117–27. DOIPubMedGoogle Scholar
- Kerdsin A, Dejsirilert S, Puangpatra P, Sripakdee S, Chumla K, Boonkerd N, Clinical and genotypic features of Streptococcus suis serotype 2 infections in humans, Thailand. Emerg Infect Dis. 2011;17:835–42.PubMedGoogle Scholar
- Blume V, Luque I, Vela AI, Borge C, Maldonado A, Dominguez L, Genetic and virulence-phenotype characterization of serotypes 2 and 9 of Streptococcus suis swine isolates. Int Microbiol. 2009;12:161–6.PubMedGoogle Scholar
- Princivalli MS, Palmieri C, Magi G, Vignaroli C, Manzin A, Camporese A, Genetic diversity of Streptococcus suis clinical isolates from pigs and humans in Italy (2003–2007). Euro Surveill. 2009;14:pii:19310.
- Lee GT, Chiu CY, Haller BL, Denn PM, Hall CS, Gerberding JL. Streptococcus suis meningitis, United States. Emerg Infect Dis. 2008;14:183–5. DOIPubMedGoogle Scholar
- Holden MT, Hauser H, Sanders M, Ngo TH, Cherevach I, Cronin A, Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS ONE. 2009;4:e6072. DOIPubMedGoogle Scholar
- Hoa NT, Chieu TTB, Nga TTT, Dung NV, Campbell J, Anh PH, Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS ONE. 2011;6:e17943. DOIPubMedGoogle Scholar
- Willenburg KS, Sentochnik DE, Zadoks RN. Human Streptococcus suis meningitis in the United States. N Engl J Med. 2006;354:1325. DOIPubMedGoogle Scholar
- Smith HE, Vecht U, Wisselink HJ, Stockhofe-Zurwieden N, Biermann Y, Smits MA. Mutants of Streptococcus suis types 1 and 2 impaired in expression of muramidase-released protein and extracellular protein induce disease in newborn germfree pigs. Infect Immun. 1996;64:4409–12.PubMedGoogle Scholar
- Chen C, Tang J, Dong W, Wang C, Feng Y, Wang J, A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS ONE. 2007;2:e315. DOIPubMedGoogle Scholar
- Ye C, Zheng H, Zhang J, Jing H, Wang L, Xiong Y, Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J Infect Dis. 2009;199:97–107. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 17, Number 12—December 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Marcelo Gottschalk, Groupe de Recherche sur les Maladies Infectieuses du Porc, Faculté de Médecine Vétérinaire, Université de Montréal, 3200 Rue Sicotte, CP5000, St-Hyacinthe, Quebec J2S 7C6, Canada
Top