Volume 18, Number 12—December 2012
Research
Borrelia, Rickettsia, and Ehrlichia Species in Bat Ticks, France, 2010
Abstract
Argas vespertilionis, an argasid tick associated with bats and bat habitats in Europe, Africa, and Asia has been reported to bite humans; however, studies investigating the presence of vector-borne pathogens in these ticks are lacking. Using molecular tools, we tested 5 A. vespertilionis ticks collected in 2010 from the floor of a bat-infested attic in southwestern France that had been converted into bedrooms. Rickettsia sp. AvBat, a new genotype of spotted fever group rickettsiae, was detected and cultivated from 3 of the 5 ticks. A new species of the Ehrlichia canis group, Ehrlichia sp. AvBat, was also detected in 3 ticks. Four ticks were infected with Borrelia sp. CPB1, a relapsing fever agent of the Borrelia group that caused fatal borreliosis in a bat in the United Kingdom. Further studies are needed to characterize these new agents and determine if the A. vespertilionis tick is a vector and/or reservoir of these agents.
Ticks are obligate hematophagous arthropods that are considered to be second only to mosquitoes as vectors of agents that cause diseases in humans (1). Ticks parasitize every class of vertebrates in most regions of the world and occasionally bite humans (1). Argas vespertilionis (also known as Carios vespertilionis) ticks parasitize several bat species around the world, except in the Americas (2,3). Bats are adequate hosts for blood ingestion by ticks because they lack dense fur and have a supply of large blood vessels just below the dermis. In addition, a larval A. vespertilionis tick was collected from a dog in Sweden (3). Common habitats for these ticks include cracks and crevices in the walls of bat-infested caves and buildings, tree holes, and any niche occupied by the host. Tick nymphs and adults can bite persons in caves (4,5). A. vespertilionis tick populations in Europe and South Africa exist in temperate climates with pronounced seasonal changes and moderate to heavy rainfall.
The role of A. vespertilionis ticks as vectors or reservoirs of bacterial, viral, or protozoal pathogens is poorly understood; however, several pathogens have been detected in these ticks. In 1966, Coxiella burnetii, the agent of Q fever, was detected in A. vespertilionis ticks collected from southern Kazakhstan (6), and in 1973, an arbovirus named Issyk-Kul virus was isolated from bats and A. vespertilionis ticks in Kyrgyzstan (7). A few years later, Issyk-Kul virus was isolated from a scientist who had become infected while conducting field work in the Kumsangir district of southern Tajikistan (8). Candidatus Babesia vesperuginis showed potential pathogenicity to a bat in the United Kingdom, and the study authors hypothesized that the A. vespertilionis tick could be a vector for these protozoa (9). Borrelia burgdorferi sensu lato, the infectious agent of Lyme disease, was detected in A. vespertilionis ticks that were collected during 1896–1994 and housed at the Natural History Museum in London. Thus, 13/13 ticks collected from bats (mostly pipistrelles) and 12 (75%) of 16 ticks collected from human dwellings had results positive for B. burgdorferi s.l. when tested by nested PCR targeting the ospA gene (10). However, PCR contaminations were not excluded for these results. It has been stated that this tick is a vector of spirochetes in bats, but no conclusive evidence has supported this hypothesis (2).
The WHO (World Health Organization) Collaborative Center for Rickettsial Diseases and other Arthropod-Borne Bacterial Diseases receives human samples and arthropod specimens from all parts of the world for tick-borne disease diagnosis. The aim of this study was to analyze A. vespertilionis ticks for the presence of Borrelia, Rickettsia, Bartonella, and Ehrlichia spp. and for C. burnetii by using molecular and culture tools.
Tick Collection
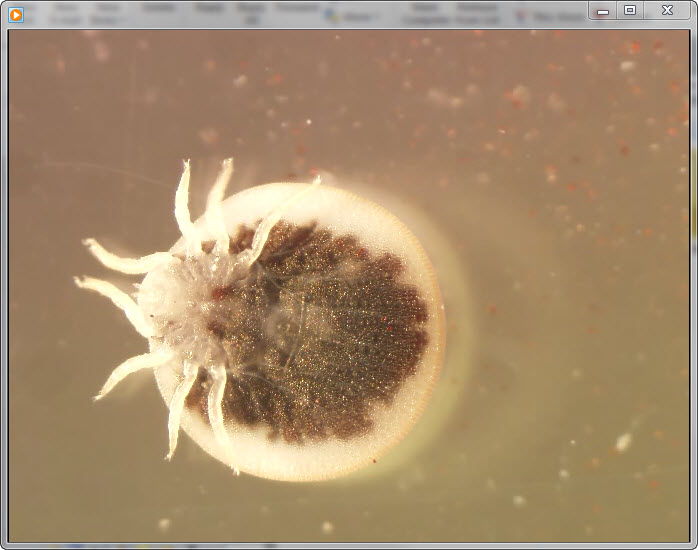
On July 12, 2010, the owners of a bat-infested home in Astien, France (42°56′18.25′′N, 1°03′54.57′′E; elevation 542 m) found 6 live ticks on the floor of their attic, which had been converted into bedrooms (Figure 1). Astien is located in southwestern France in the Ariège region of the Pyrenees Mountains. The ticks were sent to our laboratory at the WHO Collaborative Center for Rickettsial Diseases and Other Arthropod-Borne Bacterial Diseases (Marseille, France), where we used standard taxonomic keys to identify them morphologically as adult A. vespertilionis (Latreille, 1802) ticks (Video) (2).
DNA Extraction
Five of the 6 ticks were washed in a 10% water solution of commercial disinfectant–detergent (Amphomousse; Hydenet S.A., Sainghin-en-Melantois, France), rinsed in sterile water, and placed in a 1% solution of sodium hypochlorite for 10 minutes. The ticks were then rinsed with distilled water and incubated in 70% ethanol for 15 minutes, after which they were rinsed in sterile phosphate-buffered saline, dried on sterile filter paper in a laminar flow hood, and individually crushed in sterile tubes (Eppendorf; Hamburg, Germany). DNA was extracted from one half of each of the 5 ticks by using the QIAamp Tissue Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The genomic DNA was stored at 4°C until used as a template in PCR assays. The remaining portion of each tick was kept at –80°C for further analysis. DNA extraction and the molecular identification of all ticks were efficiently achieved by using one molecular system: PCR amplification with sequencing of the 338-bp region of the 12S RNA gene, as described (11). The sixth tick was kept in the tick collection of the WHO Collaborative Center for Rickettsial Diseases and other Arthropod-Borne Bacterial Diseases, Marseille, France.
Detection of Rickettsia spp.
We used quantitative real-time PCR (qPCR) with the 1029 system based on the RC0338 hypothetical protein gene to screen DNA samples from the 5 ticks for all spotted fever group (SFG) rickettsiae (12) (Table). Reactions were performed by using LightCycler 2.0 equipment and software (Roche Diagnostics GmbH, Mannheim, Germany). Master mixes were prepared according to the manufacturer’s instructions. We confirmed rickettsiae-positive results by using conventional PCR with the GeneAmp PCR System 2400 thermal cycler (PerkinElmer, Waltham, MA, USA). We used primers CS2d–CS877f and Rp CS.409p–Rp CS.1258n to amplify and sequence the full-length citrate synthase gene (gltA) found in all rickettsiae (20), and we used primers Rr. 190.70 and Rr. 190.701 to amplify and sequence a fragment of the outer membrane protein A (ompA) gene (629–632 bp), which encodes a 190-kDa protein (17). We used 2 negative controls for all PCR reactions: 1) PCR mix alone and 2) PCR mix with noninfected Rhipicephalus sanguineus tick DNA (free of Rickettsia, Ehrlichia, Anaplasma, Bartonella, and Borrelia spp. and C. burnetii). We used DNA extracted from R. montanensis as a positive control for detection of rickettsiae. Amplification products were analyzed after electrophoresis on a 1% agarose gel stained with ethidium bromide.
The second half of each tick was placed in sterile 1.5-mL plastic tubes, where they were triturated with a sterile micropestle in 600 μL of Rinaldini solution (6.8 g NaCl, 0.4 g KCl, 0.156 g NaH2PO4, 2.2 g NaHCO3, 1.0 g glucose, and 1.0 mg phenol red in 1,000 mL sterile double-distilled water). To isolate Rickettsia spp. from the tick solution, we used a shell vial cell culture assay, as described (21). In brief, we inoculated 300 µL of the rickettsia PCR–positive ticks into 7 shell vials containing 1-cm2 coverslips on which cell culture lines had been grown. Of the 7 shell vials, 3 contained a coverslip with a monolayer of mouse fibroblasts (L929 cells); 3 contained coverslips with a monolayer of human embryonic lung fibroblasts; and 1 contained a coverslip with cell line XTC-2, derived from Xenopus laevis. We did not include antimicrobial drugs in the medium. After the vials were incubated for 8, 15, and 21 days, we performed Gimenez staining and indirect immunofluorescence assays to detect rickettsial organisms in cell culture as described (21). Cultures were considered rickettsiae-positive if staining and assay results were positive. We sampled culture supernatants to identify isolates by standard PCR as described (17,20).
Detection of Borrelia spp.
We used qPCR targeting the 16S rRNA gene, as described (13), to screen DNA samples from the 5 ticks for all Borrelia spp. (Table). Samples with borreliae-positive results were confirmed positive by conventional PCR with primers Bf1-Br1 and Bor1-Bor2, which enabled amplification of the 16S rRNA gene fragment and flaB gene, respectively (18,22). We sequenced the amplified product as described above. Positive control reactions for each assay incorporated DNA extracted from Borrelia crocidurae. We injected the solution of Borrelia spp. PCR–positive ticks into 2 tubes with 10 mL of BSKH medium (Sigma-Aldrich, Taufkirchen, Germany) (23) and a 100-µL solution of antimicrobial drugs (product no. A1956 [2 mg phosphomycin (fosfomycin), 5 mg rifampin, and 250 μg amphotericin B per mL in 20% DMSO]; Sigma-Aldrich). Samples were cultivated at 33°C, and once a week we used dark-field microscopy to examine them for the presence of spirochetes. We considered samples to be negative for borreliae if no growth was detected after 8 weeks of incubation.
Detection of Bartonella spp.
We used qPCR to screen DNA samples for a fragment of the Bartonella spp. intergenic spacer region between the 16S and 23S rRNA genes (Table). Conventional PCR that amplifies a fragment of the 732-bp intergenic spacer region of Bartonella spp. was used to confirm bartonellae-positive results (15). DNA extracted from B. elizabethae served as a positive control for detection of bartonellae.
Detection of C. burnetii
We initially detected bacterial DNA by qPCR with C. burnetii–specific primers and a probe designed to amplify the IS1111 gene (14). We used qPCR with primers and a probe designed for the amplification of IS30A spacers to confirm C. burnetii–positive results (14). Amplification of both spacers indicated a positive result. In each test, DNA extracted from C. burnetii served as a positive control.
Detection of Ehrlichia/Anaplasma spp.
We detected Ehrlichia/Anaplasma spp. DNA by conventional PCR using primer set EHR16SR– EHR16SD, which amplify a 345-bp fragment of the 16S rRNA gene of ehrlichiae (16). A second PCR that amplified a fragment of the citrate synthase gene of Ehrlichia spp. was used, as described (19), to confirm positive amplification results (Table). The amplified products for both genes were sequenced as described above. Two negative controls and 1 positive control (DNA from A. phagocytophilum) were included in each test.
Sequence Analysis
All obtained sequences were assembled and edited by using Auto Assembler software version 1.4 (PerkinElmer). We analyzed sequences by using BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi) and compared them with sequences in the GenBank database. We performed multiple sequence alignments by using the ClustalX program (www.clustal.org/clustal2/). Phylogenetic trees were constructed by using the test minimum-evolution tree algorithm in the MEGA5 program (http://megasoftware.net/). Support for the tree nodes was calculated with 100 bootstrap replicates.
Tick Identification
The 12S RNA gene from 4 of the 5 A. vespertilionis ticks in our study showed 81.4% (253/311 bp) sequence similarity with the Carios capensis tick (GenBank accession no. AB075953) and 77.7% (303/390 bp) sequence similarity with the Ixodes granulatus tick (GenBank accession no. DQ003012). We submitted the 12S RNA sequence to GenBank (accession no. JX233821); no other 12S sequence for A. vespertilionis ticks was available in GenBank.
Detection of Rickettsiae spp.
Of the 5 ticks we tested, 3 (sample nos. 62494, 62497, and 62498) were positive for genus-specific rickettsiae DNA by qPCR. Subsequent sequencing of gltA gene amplicons from all positive PCR samples showed that the closest sequences available in GenBank were those for R. peacockii (accession no. CP001227), R. africae (accession no. CP001612), and R. conorii (accession no. AE006914), which showed 99.6% (1,257/1,262 bp), 99.52% (1,256/1,262 bp), and 99.52% (1,256/1,262 bp) sequence identity, respectively. The closest sequences available in GenBank for the ompA gene fragment were those for R. africae ESF-5 (accession no. CP001612), Rickettsia sp. strain S (accession no. RSU43805), and R. mongolitimonae BJ-90 strain (accession no. AF179365), which showed 99.34% (611/615 bp), 99.5% (587/590 bp), and 98.67% (594/602 bp) sequence identity, respectively. The sequences for all 3 ticks were identical.
The positive and negative controls produced the expected results. The nucleotide sequence of the full-length gltA gene of Rickettsia sp. was deposited in GenBank (accession no. JN038177) and named Rickettsia sp. AvBat. Maximum parsimony and neighbor-joining analysis of the 1,225-bp gltA gene and the presence of the ompA gene suggest that Rickettsia sp. AvBat be classified as an SFG rickettsiae, with the closest relationships to Rickettsia sp. strain S (U59735) and R. africae (U59733) (Figures 2, 3) (24). On the basis of the guidelines for the classification of new Rickettsia spp. (24), the bacterial species we identified shared <99.9% similarity with the full-length gltA gene and >98.8% similarity with the partial ompA gene.
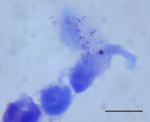
On day 15 of incubation, the shell vial cultures for 2 PCR rickettsiae-positive ticks had Gimenez staining and immunofluorescence assay results positive for Rickettsia spp. (Figure 4). The 2 isolates were established in the L929 and XTC-2 cells, respectively (3 passages/cell line).
Detection of Borrelia spp.
Of the 5 A. vespertilionis ticks tested for the presence of Borrelia sp. by qPCR, 4 were positive: tick numbers 62494, 62495, 62497, and 62498. We used standard PCR to amplify the Borrelia 16S rRNA gene fragment from all 4 positive ticks. For all samples, DNA sequence analyses of the PCR products showed 100% (1,206/1,206 bp) similarity with the 16S rRNA sequence of Borrelia sp. CPB1 (GenBank accession no. FJ868583) and 100% (736/736 bp) similarity with the flagellin gene sequence of Borrelia sp. CPB1 (GenBank accession no. FJ868584) (25). The bacterial cultures of the borrelia PCR–positive samples did not grow borreliae. Phylogenetic analysis of 2 genes (Figures 5, 6) showed that this Borrelia sp. is close to, but distinct from, a cluster containing B. recurrentis, B. duttonii, B. microtii, B. latyschewii, and B. crocidurae.
Detection of Ehrlichia/Anaplasma spp.
Five ticks were tested by standard PCR for the 16S rRNA and gltA genes specific for Ehrlichia/Anaplasma; results were positive for 3 ticks (nos. 62495, 62496, and 62497). Sequence analyses showed 99.4% (344/346 bp) similarity with the 16S rRNA gene of uncultured Ehrlichia sp. clone Khabarovsk 1931 (GenBank accession no. FJ966354) and 98.3% similarity with E. muris isolate Kh-1550 (GenBank accession no. GU358692). For these 3 ticks, the closest matches to a gltA gene fragment in GenBank were with those of Ehrlichia sp. HF (accession no. DQ647319), Ehrlichia sp. Yamaguchi (accession no. AF304145), and E. muris (accession no. AF304144), which had 88.93% (225/253 bp), 88.53% (224/253 bp), and 88.53% (224/253 bp) sequence similarity, respectively. Sequences of the 16S rRNA and gltA genes from all 3 ticks were identical.
We deposited nucleotide sequences for the 16S rRNA and gltA genes of this Ehrlichia sp. in GenBank (accession nos. JN315412 and JN315413, respectively). In phylogenetic trees based on 257 bp of the gltA gene and 348 bp of the 16S rRNA gene, this sequence is situated in the genus Ehrlichia in the E. canis group, and it is distinct from other known Ehrlichia spp. (Figures 7, 8) (26).
Detection of Bartonella spp. and C. burnetii
We tested 5 ticks by qPCR for the presence of Bartonella spp. and C. burnetii. Results were negative for these bacteria.
Co-infections
Of the 5 A. vespertilionis ticks analyzed by PCR, 4 were positive for >1 pathogen. Three of the 4 borreliae-positive ticks (nos. 62494, 62497, and 62498) were also infected with Rickettsia sp. AvBat. Two of the 3 Ehrlichia sp. AvBat–positive ticks (nos. 62495 and 62497) were also infected with Borrelia sp. CPB1, and 1 of those (no. 62497) was also infected with Rickettsia sp. AvBat.
We showed that A. vespertilionis ticks collected from a bat-infested attic in southwestern France were infected with 3 bacteria: 1) Rickettsia sp. AvBat, a new species or subspecies of the SFG rickettsiae; 2) a novel Ehrlichia sp. AvBat of the E. canis group of the genus Ehrlichia; and 3) Borrelia sp. from the relapsing fever group.
In 1956, Hoogstraal (2) reported that several A. vespertilionis ticks from Egypt were examined for rickettsiae, and all were negative. Through sequence analysis of the full-length gltA gene, we showed that the Rickettsia sp. detected in A. vespertilionis ticks in France can be classified within the SFG rickettsiae (24).
The association of Rickettsia spp. with soft ticks is poorly understood. First, intracellular rickettsia-like symbionts were described in laboratory-maintained Argas (Persicargas) arboreus ticks, but the organisms have not been shown to infect vertebrates or cause disease (27). Ornithodoros papillipes ticks that were sucking blood from guinea pigs infected with Rickettsia sibirica, the agent of North Asian tick typhus, were found to be infected with the bacterium (28). O. papillipes ticks can transmit bacteria vertically but cannot transmit it to vertebrate hosts (28). In addition, in a laboratory experiment, O. parkeri and O. rostratus ticks were infected with R. rickettsii, the agent of Rocky Mountain spotted fever, and transmitted the bacterium to a laboratory host (29). In 1974, Rehácek et al. (30) found that Argas persicus ticks collected in Armenia were massively infected with SFG group rickettsiae that were probably identical with R. slovaca, an emerging pathogen (17). Rickettsiae-infected O. moubata ticks (well-known vectors of Borrelia duttonii, an agent of tick-borne relapsing fever) were collected from human dwellings in central Tanzania and analyzed by PCR; phylogenetic analysis of the rickettsial species showed a unique cluster among the SFG rickettsiae (31). At the same time, R. felis, the agent of the so-called flea-borne spotted fever (32), was detected in 1 of 64 C. capensis ticks collected from a brown pelican rookery in South Carolina, USA (33). A rickettsial endosymbiont, later named R. hoogstraalii sp. nov., was detected by use of PCR and isolated from C. capensis ticks (34,35) collected in the United States and Japan (33,36) and, later, from Carios kelleyi bat ticks collected from residential buildings in Jackson County, Iowa, USA (37). Transstadial and transovarial transmission of this rickettsia have also been demonstrated (33). Our results support further investigation of the association of soft ticks and rickettsiae.
The sequences for the Borrelia sp. detected in this study share 100% similarity with sequences for species detected in the liver of a dead bat in the United Kingdom in 2008 (25). Phylogenetic analysis showed that the Borrelia sp. detected in the bat is close to, but distinct from, borreliae in a cluster containing B. recurrentis, B. duttonii, and B. crocidurae (25), which all cause relapsing fever in Africa (13). In the study in the United Kingdom, an A. vespertilionis larval tick (not tested for borreliae by PCR) was found attached to the infected bat, and the study authors suggested that the tick may have been the source of Borrelia infection (25). Usually, only the larvae of A. vespertilionis ticks are found on bats because they feed on their hosts from the time they are a few days old up to 2 weeks of age. Nymphs and adults become replete within 1 hour; thus, the probability is small for finding ticks in these growth stages on bats (2). In addition, adults tick may remain attached for as long as 5 hours after completed engorgement. The results of our study confirm the association between A. vespertilionis ticks and this new Borrelia sp.
The presence of ehrlichiae was detected in 3 A. vespertilionis ticks in our study, but we did not culture the samples. The association between Ehrlichia/Anaplasma spp. and soft ticks is unknown. In 1990, Ewing et al. (38) reported that an Otobius megnini tick, which detached from the ear of a child who had serologic evidence of ehrlichiosis, was negative for ehrlichia by PCR. The authors of that study attempted to use laboratory-reared O. megnini ticks to transmit E. canis, the causative agent of canine ehrlichiosis; neither transstadial nor transovarial transmission occurred.
In our study, no residents of the bat-infested attic were bitten by ticks, although 2 persons were sleeping in the room the night before the ticks were collected. Adult and nymphal A. vespertilionis ticks occasionally bite humans, and they can be highly aggressive toward humans (39). Ticks of this species have been removed from persons in Iraq, the former Soviet Union, Japan, and Africa (2,3). In Huesca Province, Spain, 2 adult A. vespertilionis ticks were found feeding on the arm of a man inhabiting a country house with many bats living in the attic (40). In 1994, Jaenson et al. (3) reported that 2 persons living near Stockholm were bitten by ticks in their bedroom; bats had been breeding in a loft above the room during May–June 1993. Severe skin reactions, fever, ulceration, erythema, and edema developed in the bitten persons, and the ulcers did not begin to cicatrise until 10 days after penicillin treatment had been initiated. Thus, medical doctors should consider bacterial infections in sick person who may have been bitten by bat ticks.
The findings from our study have repercussions for public health in many parts of Europe, Asia, and Africa because A. vespertilionis ticks have a wide geographic range and may bite humans (2). Almost any bat, whether it lives in large colonies or in small groups, may be parasitized by A. vespertilionis ticks (2). We do not know whether the novel Rickettsia sp. AvBat and Ehrlichia sp. AvBat described in our work are pathogenic for vertebrate hosts; however, the Borrelia sp. detected in these ticks was shown to be a pathogen for bat hosts (25). In addition, the Rickettsia sp. AvBat that we cultivated in a cell line should be analyzed genomically to further define the taxonomic position of this Rickettsia sp. Future studies are needed to 1) assess the role of vector and/or reservoir for each of these pathogens in A. vespertilionis, including a more precise analysis of transstadial and transovarial transmission in ticks; 2) confirm tick transmission of the bacteria in animal models; and 3) detect tick transmission of these bacteria in humans.
Dr Socolovschi is a medical doctor specializing in infectious diseases and tropical medicine at the Medical School of Marseille, France. Her research interests focus on vector-borne infectious tropical diseases and medical entomology.
Acknowledgment
We thank Sylvain Buffet for the design of the phylogenetic trees and sequence analysis.
References
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. DOIPubMedGoogle Scholar
- Hoogstraal H. African Ixodoidea: I. Ticks of the Sudan (with special reference to Equatoria Province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma). Washington (DC): US Navy; 1956. p. 1101.
- Jaenson TG, Talleklint L, Lundqvist L, Olsen B, Chirico J, Mejlon H. Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J Med Entomol. 1994;31:240–56 .PubMedGoogle Scholar
- Hoogstraal H. Notes on Egyptian ticks (Ixodoidea). I. The genus Argas (Argasidae) in the Cairo area. Proceedings of the Egyptian Academy of Sciences. 1952;7:114–27.
- Hoogstraal H. Bat ticks of the genus Argas (Ixodoidea, Argasidae). I. The subgenus Chiropterargas. Fieldiana. Zoology. 1955;37:579–600.
- Zhmaeva ZM, Pchelkina AA, Belashova VS. Spontaneous infection of Argas vespertilionis with Rickettsia burnetii in the south of Kazakhstan [in Russian]. Med Parazitol (Mosk). 1966;35:595–6 .PubMedGoogle Scholar
- Lvov DK, Karas FR, Timofeev EM, Tsyrkin YM, Vargina SG, Veselovskaya OV, “Issyk-Kul” virus, a new arbovirus isolated from bats and Argas (Carios) vespertilionis (Latr., 1802) in the Kirghiz S.S.R. Brief report. Arch Gesamte Virusforsch. 1973;42:207–9. DOIPubMedGoogle Scholar
- L’vov DK, Kostiukova MA, Pak TP, Gromashevskii VL. Isolation of an arbovirus antigenically related to Issyk-Kul virus from the blood of a human patient [in Russian]. Vopr Virusol. 1980; (
Jan–Feb ):61–2 .PubMedGoogle Scholar - Gardner RA, Molyneux DH. Babesia vesperuginis: natural and experimental infections in British bats (Microchiroptera). Parasitology. 1987;95:461–9. DOIPubMedGoogle Scholar
- Hubbard MJ, Baker AS, Cann KJ. Distribution of Borrelia burgdorferi s.l. spirochaete DNA in British ticks (Argasidae and Ixodidae) since the 19th century, assessed by PCR. Med Vet Entomol. 1998;12:89–97. DOIPubMedGoogle Scholar
- Matsumoto K, Ogawa M, Brouqui P, Raoult D, Parola P. Transmission of Rickettsia massiliae in the tick, Rhipicephalus turanicus. Med Vet Entomol. 2005;19:263–70. DOIPubMedGoogle Scholar
- Socolovschi C, Mediannikov O, Sokhna C, Tall A, Diatta G, Bassene H, Rickettsia felis–associated uneruptive fever, Senegal. Emerg Infect Dis. 2010;16:1140–2. DOIPubMedGoogle Scholar
- Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis. 2011;17:883–5. DOIPubMedGoogle Scholar
- Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Sokhna C, Bassene H, Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis. 2010;4:e654. DOIPubMedGoogle Scholar
- Raoult D, Roblot F, Rolain JM, Besnier JM, Loulergue J, Bastides F, First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol. 2006;44:278–9. DOIPubMedGoogle Scholar
- Parola P, Roux V, Camicas JL, Baradji I, Brouqui P, Raoult D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans R Soc Trop Med Hyg. 2000;94:707–8. DOIPubMedGoogle Scholar
- Sarih M, Socolovschi C, Boudebouch N, Hassar M, Parola P, Raoult D. Spotted fever group rickettsiae in ticks, Morocco. Emerg Infect Dis. 2008;14:1067–73. DOIPubMedGoogle Scholar
- Assous MV, Wilamowski A, Bercovier H, Marva E. Molecular characterization of tickborne relapsing fever Borrelia, Israel. Emerg Infect Dis. 2006;12:1740–3. DOIPubMedGoogle Scholar
- Parola P, Inokuma H, Camicas JL, Brouqui P, Raoult D. Detection and identification of spotted fever group rickettsiae and ehrlichiae in African ticks. Emerg Infect Dis. 2001;7:1014–7. DOIPubMedGoogle Scholar
- Mediannikov OY, Sidelnikov Y, Ivanov L, Mokretsova E, Fournier PE, Tarasevich I, Acute tick-borne rickettsiosis caused by Rickettsia heilongjiangensis in Russian Far East. Emerg Infect Dis. 2004;10:810–7. DOIPubMedGoogle Scholar
- Vestris G, Rolain JM, Fournier PE, Birg ML, Enea M, Patrice JY, Seven years’ experience of isolation of Rickettsia spp. from clinical specimens using the shell vial cell culture assay. Ann N Y Acad Sci. 2003;990:371–4. DOIPubMedGoogle Scholar
- Raoult D, Ndihokubwayo JB, Tissot-Dupont H, Roux V, Faugere B, Abegbinni R, Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet. 1998;352:353–8. DOIPubMedGoogle Scholar
- Houhamdi L, Raoult D. Excretion of living Borrelia recurrentis in feces of infected human body lice. J Infect Dis. 2005;191:1898–906. DOIPubMedGoogle Scholar
- Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–65. DOIPubMedGoogle Scholar
- Evans NJ, Bown K, Timofte D, Simpson VR, Birtles RJ. Fatal borreliosis in bat caused by relapsing fever spirochete, United Kingdom. Emerg Infect Dis. 2009;15:1331–3. DOIPubMedGoogle Scholar
- Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, Ray SC, Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–65. DOIPubMedGoogle Scholar
- El Shoura SM. Ultrastructure and distribution of intracellular rickettsia-like microorganisms in various organs of the laboratory-reared adult tick Argas (Persicargas) arboreus (Ixodoidea: Argasidae). Exp Appl Acarol. 1990;9:137–43. DOIPubMedGoogle Scholar
- Podboronov VM, Pchelkina AA. Characteristics of the transphase and transovarial transmission of Rickettsia sibirica by ixodid and argasid ticks [in Russian]. Med Parazitol (Mosk). 1989;4:14–8 .PubMedGoogle Scholar
- Hoogstraal H. Ticks in relation to human diseases caused by Rickettsia species. Annu Rev Entomol. 1967;12:377–420. DOIPubMedGoogle Scholar
- Rehácek J, Urvölgyi J, Kovácová E. Massive occurrence of rickettsiae of the spotted fever group in fowl tampan, Argas persicus, in the Armenian S.S.R. Acta Virol. 1977;21:431–8 .PubMedGoogle Scholar
- Cutler SJ, Browning P, Scott JC. Ornithodoros moubata, a soft tick vector for Rickettsia in east Africa? Ann N Y Acad Sci. 2006;1078:373–7. DOIPubMedGoogle Scholar
- Parola P. Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect. 2011;17:996–1000. DOIPubMedGoogle Scholar
- Reeves WK, Loftis AD, Sanders F, Spinks MD, Wills W, Denison AM, Borrelia, Coxiella, and Rickettsia in Carios capensis (Acari: Argasidae) from a brown pelican (Pelecanus occidentalis) rookery in South Carolina, USA. Exp Appl Acarol. 2006;39:321–9. DOIPubMedGoogle Scholar
- Mattila JT, Burkhardt NY, Hutcheson HJ, Munderloh UG, Kurtti TJ. Isolation of cell lines and a rickettsial endosymbiont from the soft tick Carios capensis (Acari: Argasidae: Ornithodorinae). J Med Entomol. 2007;44:1091–101. DOIPubMedGoogle Scholar
- Duh D, Punda-Polic V, Avsic-Zupanc T, Bouyer D, Walker DH, Popov VL, Rickettsia hoogstraalii sp. nov., isolated from hard- and soft-bodied ticks. Int J Syst Evol Microbiol. 2010;60:977–84. DOIPubMedGoogle Scholar
- Kawabata H, Ando S, Kishimoto T, Kurane I, Takano A, Nogami S, First detection of Rickettsia in soft-bodied ticks associated with seabirds, Japan. Microbiol Immunol. 2006;50:403–6 .PubMedGoogle Scholar
- Loftis AD, Gill JS, Schriefer ME, Levin ML, Eremeeva ME, Gilchrist MJR, Detection of Rickettsia, Borrelia, and Bartonella in Carios kelleyi (Acari: Argasidae). J Med Entomol. 2005;42:473–80. DOIPubMedGoogle Scholar
- Ewing SA, Harkess JR, Kocan KM, Barker RW, Fox JC, Tyler RD, Failure to transmit Ehrlichia canis (Rickettsiales: Ehrlichieae) with Otobius megnini (Acari: Argasidae). J Med Entomol. 1990;27:803–6 .PubMedGoogle Scholar
- Hoogstraal H. Argasid and nuttalliellid tick as parasites and vectors. Adv Parasitol. 1985;24:135–238. DOIPubMedGoogle Scholar
- Estrada-Peña A, Jongejan F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685–715. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 18, Number 12—December 2012
| EID Search Options |
|---|
|
|
|
|
|
|







Please use the form below to submit correspondence to the authors or contact them at the following address:
Philippe Parola, Unité de Recherche en Maladies Infectieuses et Tropicales Emergentes (URMITE), UMR 63, CNRS 7278, IRD 198, INSERM 1095, WHO Collaborative Center for Rickettsial Diseases and Other Arthropod-Borne Bacterial Diseases, 27 Bd Jean Moulin, 13385 Marseille Cedex 5, France
Top