Volume 18, Number 12—December 2012
Dispatch
Westward Spread of Echinococcus multilocularis in Foxes, France, 2005–2010
Abstract
During 2005–2010, we investigated Echinococcus multilocularis infection within fox populations in a large area in France. The parasite is much more widely distributed than hitherto thought, spreading west, with a much higher prevalence than previously reported. The parasite also is present in the large conurbation of Paris.
Echinococcus multilocularis is the causative agent of the parasitic zoonosis alveolar echinococcosis. The adult stage of this cestode is found mostly in the digestive tract of the red fox (Vulpes vulpes) (1). Parasite eggs, expelled in feces, are the only external living stage of the parasite life cycle. Once ingested by small mammals, they migrate to the liver and proliferate, forming protoscolices in multivesicular cysts. The life cycle is completed when a definitive host (usually canid) preys on an infected intermediate host (mostly rodent). Epidemiologic studies indicate that humans can be infected by eating raw vegetables contaminated by infected fox or dog feces or by direct contact with an infected fox or dog (2). Despite the low incidence of human alveolar echinococcosis in Europe (0.02–0.18 cases/100,000 inhabitants [3]) the zoonotic potential of the fox tapeworm, in terms of persistence and pathogenicity, poses a major parasitic threat to human health in nontropical regions (4).
Three main trends have been reported in the past decade in Europe. First, E. multilocularis prevalence has increased in foxes within areas to which it is known to be endemic (5), seemingly linked with the increase of fox population densities in Germany and Switzerland (6). Second, the geographic distribution of E. multilocularis in foxes has extended toward southern, northern, and eastern countries where it had not previously been detected; the most recent are northern Italy (7); Svalbard, Norway (8); and Sweden in 2011 (9). Third, the geographic distribution of echinococcosis has extended toward Russia and neighboring countries (10), including the Baltic states.
Until now, the eastern part of the French territory was considered the western limit of the European echinococcosis-endemic area. At the end of the 1990s, E. multilocularis in foxes was reported in only 10 of the 95 French departments (Figure 1). Studies conducted in the neighboring departments (departments 08, 21, 38, 52, 69, and 74) by sedimentation and counting technique (11) did not detect infection in foxes. However, since 1997, new cases of human echinococcosis have been recorded in areas without known infection of local fox populations (departments 01, 03, 07, 08, 12, 21, 23, 31, 35, 61, 44, 59, 61, 76, and 95) (2).
We present the results of a large-scale survey of E. multilocularis infection in foxes in France. Our study was conducted in 42 departments covering an area of 239,178 km2 representing almost all of northeastern France.
During 2005–2010 (time span needed to cover the study area) and during the months more favorable for infection (October–April [3]), foxes were either shot at night or trapped. The sampling size was chosen to collect ≈100 foxes from each department. Therefore, a grid of 5 km × 5 km to 10 km × 10 km, depending on the department size, was superimposed over the sampling area, and no more than 1 fox was collected in each square. The geographic district where the sample was taken was then noted, and each fox was randomly allocated geographic coordinates within the commune (a French administrative division of 10–100 km2).
Adult E. multilocularis worms were identified in departmental veterinary laboratories. Staff were trained by the Anses-Nancy laboratory (National Reference Laboratory for echinococcoses); that laboratory also confirmed any unrecognized specimens. For time- and cost-effectiveness during the analysis, we used the segmental and sedimentation counting technique (12).
We used the χ2 test to compare E. multilocularis prevalence between departments. The distribution of E. multilocularis prevalence in foxes was modeled against geographic coordinates by using a generalized additive model with a logistic link function and a thin plate regression spline on 300 knots (13). Analyses and graphic displays were conducted by using ArcGIS 9.3, R 2.14.0 and the R packages maptools 0.8–10, mgcv 1.7–12, sp. 0.9–91, and splancs 2.01–29.
A total of 3,307 foxes were collected (Table 1). Eighty-five could not be assigned a commune code and were not kept for further analysis, except to compute E. multilocularis prevalence in departments. The mean number of foxes collected by department was 84.95 (± SD 25.76), which represents a mean of 1.56 foxes per 100 km2 (± SD 0.57). For 4 departments, (36, 61, 67, and 69), full sampling could not be completed because of technical and/or administrative reasons. Urban areas, such as departments 93, 95, and 91, also were undersampled because of human population density and high urbanization, all factors preventing easy fox sampling.
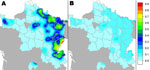
We confirmed E. multilocularis in foxes in 35 departments (Figure 2). The prevalence varied widely among departments, from 0 (95% CI 0–5%) to 54% (95% CI 42%–64%) (Table 1) but was locally higher in some areas (Figure 2). The mean prevalence in the entire studied area was 17% (n = 3,307; 95% CI 16%–19%). The prevalence in the historically echinococcosis-endemic area was 41% (n = 789; 95% CI 37%–44%) and represented >55% of all infected foxes and <21% of the total area studied. Furthermore, in comparing our results with those of earlier similar studies during the same season with the same technique, we detected a significant increase of E. multilocularis prevalence in foxes over time in most of these departments (Table 2).
Our study confirms the presence of E. multilocularis in areas where it is known to be endemic and indicates its presence in 25 additional departments. However, we cannot discard the possibility that E. multilocularis was present but remained undetected during the 1980s–1990s. That E. multilocularis could have remained undetected if it were not already at a very low prevalence in general is doubtful. Isolated human cases recorded in the early 2000s outside areas to which it is known to be endemic corroborate this possibility (3). The same uncertainty applies in other parts of Europe (14). Taken as a whole, these findings indicate that the transmission intensity of E. multilocularis through fox populations in the occidental part of the European focus area is likely to have increased during the late 1990s and led to a much higher average prevalence than previously reported. Furthermore, infected foxes close to large-scale conurbations, such as Paris and its large suburban surrounding departments (93, 91, and 77) (Figure 1) amounting to 11,728,240 inhabitants, may create new conditions for human exposure similar to those already described in other highly urbanized cities, such as in Switzerland, Germany, and eastern France (Nancy), but on a much larger scale.
We believe that the public needs to be proactively informed and protected, including through awareness initiatives among urban residents and, in specific areas (15), more direct action toward the parasite may be considered. Monitoring the possible further extension of the parasite westward and southward and the evolution of prevalence in foxes in the historically and the newly echinococcosis-endemic areas also are essential.
Dr Combes is head of the Entente for the Control of Zoonoses, Nancy, France. His research interests include epidemiologic surveillance and control of zoonoses.
Acknowledgment
We thank the wildlife and game associations for the fox sampling and the departmental veterinary laboratories for the analyses of fox intestines.
References
- Kapel CMO, Torgerson PR, Thompson RCA, Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol. 2006;36:79–86. DOIPubMedGoogle Scholar
- Grenouillet F, Knapp J, Millon L, Raton V, Richou C, Piarroux M, L’échinococcose alvéolaire humaine en France en 2010. Bulletin Épidémiologique Hebdomadaire. 2010;38:27.
- Giraudoux P, Raoul F. Écologie du paysage, perturbations anthropogéniques et transmission de l’échinocoque alvéolaire. In: Gauthier-Clerc M, Thomas F. editors. Ecologie de la Santé et Biodiversité. Paris: De Boeck; 2010. p. 211–26.
- Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. DOIPubMedGoogle Scholar
- Romig T, Dinkel A, Mackenstedt U. The present situation of echinococcosis in Europe. Parasitol Int. 2006;55(Suppl):S187–91. DOIPubMedGoogle Scholar
- Deplazes P, Hegglin D, Gloor S, Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:7–84. DOIPubMedGoogle Scholar
- Casulli A, Bart JM, Knapp J, La Rosa G, Dusher G, Gottstein B, Multi-locus microsatellite analysis supports the hypothesis of an autochthonous focus of Echinococcus multilocularis in northern Italy. Int J Parasitol. 2009;39:837–42. DOIPubMedGoogle Scholar
- Fuglei E, Stien A, Yoccoz NG, Ims RA, Eide NE, Prestrud P, Spatial distribution of Echinococcus multilocularis, Svalbard, Norway. Emerg Infect Dis. 2008;14:73–5. DOIPubMedGoogle Scholar
- Osterman LE, Juremalm M, Christensson D, Widgren S, Hallgren G, Ågren EO, First detection of Echinococcus multilocularis in Sweden, February to March 2011. Euro Surveill. 2011;16:pii:19836.
- Sikó SB, Deplazes P, Ceica C, Tivadar CS, Bogolin I, Popescu S, Echinococcus multilocularis in south-eastern Europe (Romania). Parasitol Res. 2011;108:1093–7. DOIPubMedGoogle Scholar
- Depaquit J, Gallego A, Usseglio F, Liance M, Favriel JM. L’échinococcose alvéolaire dans le département français des Ardennes: cas isolés ou nouveau foyer? Parasite. 1998;5:285–7.PubMedGoogle Scholar
- Umhang G, Woronoff-Rhen N, Combes B, Boue F. Segmental sedimentation and counting technique (SSCT): an adaptable method for qualitative diagnosis of Echinococcus multilocularis in fox intestines. Exp Parasitol. 2011;128:57–60. DOIPubMedGoogle Scholar
- Wood SN. Generalized additive models: an introduction with R. London: Chapman & Hall/CRC; 2006.
- Lucius R, Bilger B. Echinococcus multilocularis in Germany: increased awareness or spreading of a parasite? Parasitol Today. 1995;11:430–4. DOIPubMedGoogle Scholar
- Hegglin D, Deplazes D. Control strategy for Echinococcus multilocularis. Emerg Infect Dis. 2008;14:1626–8. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 18, Number 12—December 2012
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Patrick Giraudoux, Chrono-environment, University of Franche-Comte, place Leclerc, 25030 Besançon Cedex, France
Top