Volume 18, Number 5—May 2012
Dispatch
Origin of Human T-Lymphotropic Virus Type 1 in Rural Côte d’Ivoire
Cite This Article
Citation for Media
Abstract
Simian T-lymphotropic virus type 1 (STLV-1) strains occasionally infect humans. However, the frequency of such infections is unknown. We show that direct transmission of STLV-1 from nonhuman primates to humans may be responsible for a substantial proportion of human T-lymphotropic virus type 1 infections in rural Côte d’Ivoire, where primate hunting is common.
Human T-lymphotropic virus type 1 (HTLV-1) can induce adult T-cell leukemia or lymphoma and HTLV-1–associated myelopathy or tropical spastic paraparesis. These pathologies are a serious threat to the several million persons infected with HTLV-1 (1).
Although HTLV-1 has spread globally, its geographic distribution is not uniform. Most infected persons live in areas where the virus is endemic and seroprevalence is comparatively high (>1%) (1), i.e., in Japan, Melanesia, South America, the Caribbean, and sub-Saharan Africa. Phylogenetic analyses demonstrate that the geographic distribution of HTLV-1 genetic diversity is also not uniform. The most genetic diversity is seen in sub-Saharan Africa, where 6 of the 7 human molecular subtypes (HTLV-1A, B, D, E, F, and G) are found. Of those 6 subtypes, 5 are mainly found in or endemic to central Africa: HTLV-1B, D, E, F, and G (1).
Molecular HTLV-1 subtypes from humans in central Africa belong to composite clades that comprise HTLV-1 strains and simian T-lymphotropic virus type 1 (STLV-1) strains derived from nonhuman primates (2). Nonhuman primates in Africa are considered to be the source of recurrent zoonotic transmissions of STLV-1 to local human populations; virus transmission is believed to occur during the collection and consumption of nonhuman primate bushmeat. This belief is supported by the fact that self-reported nonhuman primate hunters in Cameroon were infected with viruses closely related to STLV-1 strains circulating among local nonhuman primate prey (3). However, because intrafamilial transmission of HTLV-1B and -1D was also documented among hunters-gatherers in Cameroon (4), it is impossible to sort out cases of direct zoonotic transmission of STLV-1 from cases of consecutive human-to-human spread of virus (evolutionary rates for HTLV-1/STLV-1 are very slow) (5).
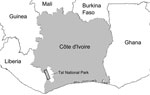
However, HTLV-1 and STLV-1 strains from western African segregate clearly in phylogenetic analyses; most humans are infected with HTLV-1A, the only human-restricted molecular subtype (6–9). Therefore, in western compared with central Africa, human infections with viruses closely related to local STLV-1 strains are much more likely to reflect direct zoonotic transmission. This situation enabled us to investigate the frequency of such direct zoonotic transmissions in a rural region of Côte d’Ivoire neighboring the Taï National Park (Figure 1).
During 2006–2007, blood samples were obtained from 776 volunteers living in 18 villages bordering Taï National Park. All participants signed informed consent forms and completed questionnaires aimed at determining their exposure to nonhuman primate bushmeat through activities such as hunting of nonhuman primates or consumption of nonhuman primate bushmeat.
To determine effective exposure to HTLV-1/STLV-1, we used an HTLV-1/2 ELISA to test serum samples for reactivity to HTLV-1/2 antigens (10). Of the 776 serum samples, 16 were positive according to the ELISA manufacturer’s criteria; an additional 15 samples had values just below the cutoff. We extracted DNA from all ELISA-reactive samples and performed a search for HTLV-1/STLV-1 sequences by using a tax-specific quantitative PCR (8). Of the 31 samples, 10 were positive and were analyzed by using a multiplex nested/seminested PCR targeting env and long terminal repeat (LTR) sequences (Table 1; Technical Appendix). To identify multiple infections with HTLV-1/STLV-1, this assay was applied on near endpoint dilutions of the 10 DNA extracts (2–6 starting template molecules per reaction; Technical Appendix). For each person, 6–20 env and 2–20 LTR sequences (15–40 sequences per person) were determined by Sanger sequencing. No evidence of multiple infections was found.
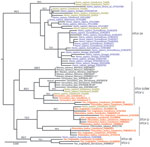
Phylogenetic analyses were performed by using Bayesian and maximum likelihood methods on env and LTR datasets (Technical Appendix). Both methods agreed on all essential features of the LTR tree topology (Figure 2) and env tree topology (Technical Appendix Figure). Six of the newly determined HTLV-1 sequences were unambiguously related to HTLV-1A (bootstrap, 94; posterior probabilities, 1) (Figure 2), confirming the predominance of this molecular subtype in Côte d’Ivoire and in western Africa (6). Another 3 HTLV-1 sequences were closely related to STLV-1 sequences found in sooty mangabeys (Cercocebus atys) from Taï National Park (bootstrap, 83; posterior probabilities, 1) (Figure 2; Technical Appendix Table 2), whereas the last 1 was related to STLV-1 sequences from red colobus monkeys (Piliocolobus badius badius) and chimpanzees (Pan troglodytes verus) from Taï National Park (bootstrap, 98; posterior probabilities, 1) (Figure 2; Technical Appendix Table 2) (7,8). Bayesian analyses were run under the assumption of a molecular clock and calibrated. However, reliable divergence dates could not been determined because most shallow nodes of the trees, including those of interest here, were not supported (Technical Appendix). Observed divergences, however, seemed compatible with cross-species transmission events, particularly in the case of study participant Pau009 (divergence to closest STLV-1, 0% in LTR and 0.2% in env) (Table 1).
We investigated the frequency of direct zoonotic transmission of STLV-1 in a rural region of Côte d’Ivoire neighboring Taï National Park and found that only 2 of the STLV-1–related sequences would be compatible with a local human-to-human transmission (Gah050 and Kei005; Figure 2). Therefore, our data support the notion that direct zoonotic transmissions of STLV-1 represent a measurable proportion of HTLV-1 infections, at least in rural regions bordering nonhuman primate habitat. In addition, these results mirror observations made among adult chimpanzees from Taï National Park, which are often infected with retroviruses (i.e., simian foamy viruses and STLV-1) of their prey (Figure 2) (7,11).
Despite the high prevalence of STLV-1, simian foamy virus, and simian immunodeficiency virus infections among red colobus populations (8) and the fact that this nonhuman primate species is the one most frequently hunted by humans (4), most zoonotic transmissions of retroviruses in western Africa seem to originate from sooty mangabeys, as shown here for STLV-1 and previously described for simian immunodeficiency virus of sooty mangabeys, the precursor of HIV-2 (12). It remains to be determined whether these zoonotic transmissions from sooty mangabeys are favored as a result of molecular determinants (e.g., convergent evolution of retroviral receptors) or behavioral determinants (e.g., increased aggressiveness).
Considering the human exposure to nonhuman primate bushmeat in this region (as illustrated by ≈150,000 kg sold per year in markets) (13) and given the high prevalence of STLV-1 among local nonhuman primates (7,8), the observation that zoonotic transmission events are, in absolute terms, exceedingly rare is striking. Yet, the accumulation of genetically distinct HTLV-1/STLV-1 over restricted geographic areas remains possible, as illustrated by the finding of 1 person infected with HTLV-1A and 2 persons infected with putative STLV-1 in a single village, Kéibly (Table 1; Figure 1). Such local accumulations add to the threat represented by direct transmissions of STLV-1 because they can provide an opportunity for recombinant viruses to emerge, even though HTLV-1/STLV-1 biology may be unfavorable to recombination (14).
The analysis of behavioral data reveals generalized exposure of local populations to cooked nonhuman primate bushmeat (Table 2). Exposure to fresh tissues, which can be expected to be more risky in terms of retroviral transmission, is less common (Table 2). Along a gradient of bushmeat freshness, going from hunting to preparation and cooking, a clear reversal of sex-related skew can be observed: only men are hunters, men and women are equally involved in dismembering, and women predominantly prepare and cook nonhuman primate bushmeat (Table 2). Hence, men likely constitute a population at risk. In our study, 75% of persons who were identified as infected with viruses closely related to STLV-1 were men, whereas all HTLV-1A–infected persons were women. Increased surveillance for zoonotic transmission of STLV-1 to humans in areas where such transmission is more likely and increased surveillance of nonhuman primate species with high transmission potential (like sooty mangabeys) will contribute to a better understanding of risk factors.
Dr Calvignac-Spencer is a researcher at the Robert Koch-Institut. His research interest is in the patterns of viral transmission in the wild between and within primate species.
Acknowledgments
We thank the authorities in Côte d’Ivoire for long-term support, especially the Ministry of Environment and Forests and the Ministry of Research, the directorship of the Taï National Park, the Office Ivoirien des Parcs et Réserves, and the Swiss Research Center in Abidjan. We also thank the Taï Chimpanzee Project for logistic support and S. Metzger, field assistants, and students for assistance in sample collection. We warmly thank Ulla Thiesen for her efficient assistance in the laboratory, Sandra Junglen and Sabrina Weiß for helpful discussions, and Daniel Driscoll for proofreading.
This work was supported by the Deutsche Forschungsgemeinschaft (grant LE1813/4-1) and the Robert Koch-Institut.
References
- Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–81. DOIPubMedGoogle Scholar
- Van Dooren S, Verschoor EJ, Fagrouch Z, Vandamme AM. Phylogeny of primate T lymphotropic virus type 1 (PTLV-1) including various new Asian and African non-human primate strains. Infect Genet Evol. 2007;7:374–81. DOIPubMedGoogle Scholar
- Wolfe ND, Heneine W, Carr JK, Garcia AD, Shanmugam V, Tamoufe U, Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc Natl Acad Sci U S A. 2005;102:7994–9. DOIPubMedGoogle Scholar
- Calattini S, Betsem E, Bassot S, Chevalier SA, Tortevoye P, Njouom R, Multiple retroviral infection by HTLV type 1, 2, 3 and simian foamy virus in a family of Pygmies from Cameroon. Virology. 2011;410:48–55. DOIPubMedGoogle Scholar
- Lemey P, Pybus OG, Van Dooren S, Vandamme AM. A Bayesian statistical analysis of human T-cell lymphotropic virus evolutionary rates. Infect Genet Evol. 2005;5:291–8. DOIPubMedGoogle Scholar
- Diop S, Calattini S, Abah-Dakou J, Thiam D, Diakhate L, Gessain A. Seroprevalence and molecular epidemiology of human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 in blood donors from Dakar, Senegal. J Clin Microbiol. 2006;44:1550–4. DOIPubMedGoogle Scholar
- Junglen S, Hedemann C, Ellerbrok H, Pauli G, Boesch C, Leendertz FH. Diversity of STLV-1 strains in wild chimpanzees (Pan troglodytes verus) from Côte d’Ivoire. Virus Res. 2010;150:143–7. DOIPubMedGoogle Scholar
- Leendertz SAJ, Junglen S, Hedemann C, Goffe A, Calvignac S, Boesch C, High prevalence, coinfection rate, and genetic diversity of retroviruses in wild red colobus monkeys (Piliocolobus badius badius) in Taï National Park, Côte d’Ivoire. J Virol. 2010;84:7427–36. DOIPubMedGoogle Scholar
- Zehender G, Ebranati E, De Maddalena C, Gianelli E, Riva A, Rusconi S, Description of a “trans-Saharan” strain of human T-lymphotropic virus type 1 in West Africa. J Acquir Immune Defic Syndr. 2008;47:269–73. DOIPubMedGoogle Scholar
- Leendertz FH, Boesch C, Ellerbrok H, Rietschel W, Couacy-Hymann E, Pauli G. Non-invasive testing reveals a high prevalence of simian T-lymphotropic virus type 1 antibodies in wild adult chimpanzees of the Taï National Park, Côte d’Ivoire. J Gen Virol. 2004;85:3305–12. DOIPubMedGoogle Scholar
- Leendertz FH, Zirkel F, Couacy-Hymann E, Ellerbrok H, Morozov VA, Pauli G, Interspecies transmission of simian foamy virus in a natural predator–prey system. J Virol. 2008;82:7741–4. DOIPubMedGoogle Scholar
- Santiago ML, Range F, Keele BF, Li Y, Bailes E, Bibollet-Ruche F, Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Taï Forest, Côte d’Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J Virol. 2005;79:12515–27. DOIPubMedGoogle Scholar
- Refisch J, Koné I. Impact of commercial hunting on monkey populations in the Taï region, Côte d’Ivoire. Biotropica. 2005;37:136–44. DOIGoogle Scholar
- Wattel E, Vartanian J, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–8.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 18, Number 5—May 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Fabian H. Leendertz; Research Group Emerging Zoonoses, Robert Koch-Institut, Nordufer 20, 13353 Berlin, Germany
Top