Volume 19, Number 1—January 2013
Research
Infections with Spore-forming Bacteria in Persons Who Inject Drugs, 2000–2009
Abstract
Since 2000 in the United Kingdom, infections caused by spore-forming bacteria have been associated with increasing illness and death among persons who inject drugs (PWID). To assess temporal and geographic trends in these illnesses (botulism, tetanus, Clostridium novyi infection, and anthrax), we compared rates across England and Scotland for 2000–2009. Overall, 295 infections were reported: 1.45 per 1,000 PWID in England and 4.01 per 1,000 PWID in Scotland. The higher rate in Scotland was mainly attributable to C. novyi infection and anthrax; rates of botulism and tetanus were comparable in both countries. The temporal and geographic clustering of cases of C. novyi and anthrax into outbreaks suggests possible contamination of specific heroin batches; in contrast, the more sporadic nature of tetanus and botulism cases suggests that these spores might more commonly exist in the drug supply or local environment although at varying levels. PWID should be advised about treatment programs, injecting hygiene, risks, and vaccinations.
Clostridium and Bacillus spp. produce spores that can be found in soil, dust, human and animal intestines, and aquatic environments; these spores can remain viable for long periods (1). Spores can contaminate illicit drugs or drug-injecting equipment. If injected intravenously, intramuscularly, or subcutaneously, spores can germinate and produce potent neurotoxins or histotoxins that cause illness and death (2). In persons who inject drugs (PWID), these organisms often initially cause localized infections; however, the toxins they produce can result in severe systemic illness, which usually becomes apparent within a week after infection.
Infections with spore-forming bacteria in PWID have historically been more common in the United States than in Europe. By the 1950s, injection drug use accounted for most cases of tetanus in New York (3,4), and wound botulism associated with injecting black tar heroin was first described in California just over 2 decades ago (5). In contrast, such infections have occurred more recently in Europe; in the United Kingdom, for example, few infections had been reported before 2000 (1). Nevertheless, a recent article noted that 367 infections with spore-forming bacteria among PWID in Europe were reported during 2000–2009 (6). Although high rates of these infections were reported in northwestern Europe (United Kingdom, Norway, and Ireland), few cases have been reported elsewhere in Europe. The reasons for this marked regional variation within Europe remain unclear but might reflect drug trafficking routes, the type of drugs injected locally, and/or differences in local injecting practices (6).
In addition to the varied extent of these infections among PWID across Europe, some regional variation within the United Kingdom has been noted (7) but not fully explored. To further explore this variation, we compared the regional rates of infection and death caused by a small number of aerobic and anaerobic spore-forming bacteria among PWID in Scotland and England over a 10-year period beginning in 2000. The availability of detailed epidemiologic data on cases in England and Scotland enabled us to examine regional and temporal trends and demographic patterns. Information about differences in drug-injecting populations and practices that might be associated with infection could be used to prevent future infections.
Case Ascertainment
We collated information about reported cases of infection with Clostridium botulinum (botulism), C. tetani (tetanus), C. novyi, and Bacillus anthracis (anthrax) among PWID in England and Scotland with dates of onset from January 2000 through December 2009. Information about suspected cases of botulism or tetanus was obtained from voluntary or statutory notifications to the Health Protection Agency and Health Protection Scotland; reports included information about possible risk factors. Corresponding samples were sent to the Foodborne Pathogens Reference Unit, the Special Pathogens Reference Unit, or the Anaerobic Reference Laboratory for the detection of toxin and microbiological confirmation. Confirmation criteria have been described (8,9). Clinical, demographic, and risk factor information was obtained from a questionnaire administered to patients by clinicians or microbiologists. Information about cases of C. novyi infection and anthrax were obtained from reports and documentation of the respective outbreaks (7,10–12); case definitions are described in these reports. The analyses presented here are limited to definite and probable C. novyi infections and confirmed anthrax cases.
Data Analysis
To derive infection rates, we used regional estimates of the number of PWID in England (2004–05 fiscal year) and Scotland (2006), closest to the midpoint of the 10-year period (2000–2009) (13,14). Both sets of estimates of PWID populations were derived by log–linear modeling of capture–recapture data. Numbers of infections were tabulated by region (England) and National Health Service Board area (Scotland), and rates per 1,000 PWID were calculated.
Numbers of infections were also tabulated by sex, and median age of case-patients was calculated. To compare demographics, we compared the sex distribution and median age of our study population with that derived from national surveys of PWID in England and Scotland (these data were not available from the capture–recapture PWID estimates described above) undertaken in years closest to the midpoint of the 10-year period. For England, we used data from the 2005 Unlinked Anonymous Monitoring Survey of PWID (15) and, for Scotland, the 2008–2009 Needle Exchange Surveillance Initiative (16). These 2 surveys aimed to recruit representative samples of PWID in contact with specialist services; the numbers of PWID participating in these surveys who had injected in the preceding 4 weeks were 1,740 and 1,772, respectively. We compared national survey respondents and case-patients in terms of sex and age by using χ2 tests (or Fisher exact tests when there were <5 persons in a given tabular cell) and Wilcoxon rank tests, respectively.
During January 1, 2000–December 31, 2009, a total of 295 infections caused by spore-forming bacteria (157 botulism, 33 tetanus, 92 C. novyi, and 13 anthrax) were reported among PWID in England and Scotland; the overall infection rate was 1.83 cases per 1,000 PWID. Two thirds (199) of these cases were reported in England and one third (96) in Scotland, corresponding to rates of 1.45 and 4.01 per 1,000 PWID, respectively (Table 1).
The number of reported cases varied over time (Figure 1). The C. noyvi infections and anthrax cases were clustered in 2000 and 2009, respectively, and most tetanus cases occurred during 2003–2005. By contrast, botulism was reported in all years; the annual number of cases varied from 3 to 41.
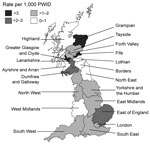
Infection rates varied by health region. In England, rate of infection varied from 0.68 cases per 1,000 PWID for the West Midlands to 2.02 for the East of England (Figure 2); rates were also high for the East Midlands, London, and the North West (1.7, 1.9, and 1.7 cases/1,000 PWID, respectively). In Scotland, rates ranged from zero in 3 rural areas with small populations of PWID (Ayrshire and Arran, Borders, and Highlands) to 7.7 per 1,000 PWID in Greater Glasgow and Clyde; rates were also high in Grampian (3.6 cases/1,000 PWID) and Fife (3.9 cases per /1,000 PWID).
In terms of specific infections, the rate of botulism was slightly higher for England than for Scotland, although this difference was not statistically significant (1.0 vs. 0.8 cases/1,000 PWID, p = 0.232), and rates of tetanus were similar for both countries (0.20 vs. 0.21/1,000 PWID, p = 0.962). In contrast, rates of C. novyi infections and anthrax were markedly higher for Scotland than for England (2.5 vs. 0.2 cases/1,000 PWID, p<0.001; and 0.5 vs. 0 cases/1,000 PWID, p<0.001, respectively). C. novyi infections were particularly concentrated in Greater Glasgow and Clyde (5.6 cases/1,000 PWID) and in the North West region of England (0.7 cases/1,000 PWID). Higher than average rates of botulism were reported in the East of England region (1.8 cases/1,000 PWID) and in Grampian (2.0 cases/1,000 PWID).
When we compared the demographic characteristics of case-patients with those of PWID participating in the 2 national surveys, we found that the proportion of female patients with tetanus, C. novyi infection, and anthrax was higher (38%–60%) than the proportion of female PWID in the community (24%–26%) (Table 2). These differences were statistically significant for C. novyi infections in England and Scotland (p = 0.011 and p<0.001, respectively) and for tetanus cases in England. In England, the median age of PWID with botulism, tetanus, and C. novyi infection ranged from 33 to 37 years; this age range was higher among those with botulism than among the PWID participating in the Unlinked Anonymous Monitoring Survey (37 vs. 32 years; p<0.001). In Scotland, the median ages of PWID with botulism, C. novyi infection, and anthrax were comparable to the median age of PWID from the community sample; whereas, the median age was higher for PWID infected with tetanus (47 vs. 33 years), although not significantly so (p = 0.065).
Of the 295 reported case-patients, 52 (18%) are known to have died. Of these, 8 (5%) died of botulism, 2 (6%) died of tetanus, 36 (39%) died of C. novyi infection, and 6 (46%) died of anthrax.
Over the decade beginning in 2000, almost 300 severe infections caused by spore-forming bacteria were reported among PWID in England and Scotland; 52 of these patients died. The distribution of the cases varied markedly between these countries. In Scotland, the number of cases was excessive relative to the estimated population of PWID when compared with England; this excess, however, is mainly attributable to an excess of C. novyi infections and anthrax cases. In contrast, rates of botulism and tetanus for Scotland were lower than and comparable with, respectively, those for England.
In the United Kingdom, microbiological testing has usually been unable to confirm the presence of these bacterial species in seized or surrendered heroin (2), although, in 2009, C. botulinum was isolated from 1 sample of heroin seized in Scotland (K.A. Grant, pers. comm.). Nevertheless, it is generally recognized that the infections discussed here have resulted from contaminated heroin, which might have become contaminated during processing, transport, or storage. In the United Kingdom, 90% of heroin used originates in Afghanistan, where the opium is produced and—increasingly since 2002—converted to heroin. Heroin from Afghanistan usually travels over land, passing through several countries before entering the European Union and reaching the United Kingdom (17,18). The conditions in which heroin is processed, transported, and stored are uncertain; because these activities are illegal, they all probably make the drug vulnerable to inadvertent contamination with bacterial spores, for example, from soil or dust. Contaminated heroin is thought to have been the source of B. anthracis infection in a drug injector in Norway in 2000 (19,20) and in the more recent outbreak among PWID in Europe (12). Another source of potential contamination is drug adulterants (cutting agents), which are widely used to dilute and increase the bulk of illicit drugs (21). Although most infections probably resulted from upstream (before it reaches the end user) contamination of heroin, spores on the soiled hands of users and dirty needles could be inoculated during the injection process (22). This mode of infection remains unproven, although signs of tetanus were observed by Arthur Nicolaier in 1884 after he injected garden soil containing C. tetani (at that point unnamed) into animals (23), and clostridial infections after injection through dirt-covered hides have been reported (24).
Although the presence of bacterial spores is a necessary prerequisite for infection, several other factors might influence the development and geographic patterns of infections. The clustering of cases of C. novyi infection; anthrax; and, to a lesser extent, tetanus into outbreaks suggests that the contamination might have affected specific batches of heroin. By contrast, the botulism cases were generally more sporadic (albeit with some clustering) (25,26), suggesting that C. botulinum and, to a lesser degree, C. tetani spores might be more commonly present in the drug supply or in the local environment but at varying levels of contamination. Different drug supply routes serving eastern and western England and Scotland (12) might account for some of the geographic patterns and are consistent with the excessive C. novyi infections among PWID in Greater Glasgow and Clyde (western Scotland) and the North West region of England and with the higher rates of botulism among PWID in the East of England and Grampian (eastern Scotland).
Practices such as skin or muscle popping (intentionally or accidentally injecting into skin or muscle) (10,27,28) or the use of large amounts of citric acid to dissolve heroin can damage soft tissue, leading to necrosis and providing a suitable environment for anaerobic bacteria, such as Clostridium spp., to thrive. Older age (a proxy for a longer injecting career) and female sex have been associated with infections and injuries at injecting sites (29,30), which are associated with difficulty accessing veins. These persons might resort to injecting into the skin or muscle. Geographic variation in these practices might explain some of the variations seen in this study. PWID across England and Scotland might be regularly exposed to botulism and tetanus spores, but the levels of infection might be higher in some areas where skin or muscle popping is more common. This finding is consistent with the high proportion of women and the older median age among PWID with clostridial or B. anthracis infections described here in comparison with the wider population of PWID in England and Scotland. The emergence of these infections as a major public health issue in the United Kingdom and Ireland (6) over the past decade might reflect the changing characteristics of the drug-using population, an aging cohort of users resulting from the marked increase in injection drug use during the 1980s and 1990s (31). With regard to tetanus, variation could also reflect differences in the levels of effective immunization among PWID.
This analysis captures only the anthrax cases reported before the end of December 2009; however, the anthrax outbreak continued into 2010 and resulted in a total of 52 confirmed cases, including 5 in England (12,32). The risk factors for anthrax might differ from those for the other infections/diseases because anthrax is the only disease considered here that is caused by an aerobic bacterium. Furthermore, we cannot exclude the possibility of inhalational anthrax in some of the case-patients who reported smoking heroin (11,12). We considered only confirmed cases of anthrax in this analysis; however, the inclusion of probable cases (although it would have increased the numbers and rates) most likely would not have changed our findings with regard to demographic characteristics, given that probable and confirmed cases were similar in terms of age (mean 34 vs. 35 years, respectively) and sex (29% vs. 30% female, respectively) (12).
Because infections might go unreported or be misdiagnosed, the data presented here potentially underestimate the actual numbers of infections among PWID in England and Scotland. For tetanus and botulism, little toxin is required to cause symptoms; therefore, in combination with a reported history of injection drug use, index of clinical suspicion should be high (33–35). However, tetanus cases are underreported because some clinicians are not familiar with this rare disease (36). Misdiagnosis of infection might also account for underreporting because the symptoms of other illnesses can resemble those of the infections of interest in this study (e.g., Guillain-Barré syndrome vs. botulism) (33,34). In addition, if an injection site infection is treated promptly with broad spectrum antimicrobial drugs before tissue samples are collected, microbiological confirmation might not be possible (7).
Another limitation of this study is associated with estimates of the size of the PWID population. The estimates from Scotland and England were produced by using indirect methods by the same team but were based on different data sources and definitions. Moreover, estimates produced by indirect methods are difficult to validate. For example, the national study used here estimated 17,909 PWID in London (13), but another study estimated >30,000 PWID in London for 2000–2001 (37).
Because the quality and safety of illicit heroin is not monitored or controlled, sporadic cases and outbreaks of illness associated with spore-forming bacteria among PWID might continue. Persons who use heroin should be encouraged to seek treatment for their dependency. Health care professionals should educate PWID who continue to inject about injecting hygiene, the risks from specific injecting practices that have been associated with these infections, the need to ensure that their tetanus vaccinations are up to date, and the need to seek care if they have symptoms of an injection-related infection. Public health professionals should continue to be vigilant to ensure prompt detection of outbreaks and so permit the rapid dissemination of advice.
Ms Palmateer is an epidemiologist with Health Protection Scotland. Her work focuses on the epidemiology and prevention of bacterial and viral infections, primarily hepatitis C virus infections, among PWID.
Acknowledgment
We thank Avril Taylor for providing data from the Needle Exchange Surveillance Initiative.
References
- Brett MM, Hood J, Brazier JS, Duerden BI, Hahne SJ. Soft tissue infections caused by spore-forming bacteria in injecting drug users in the United Kingdom. Epidemiol Infect. 2005;133:575–82. DOIPubMedGoogle Scholar
- McLauchlin J, Mithani V, Bolton FJ, Nichols GL, Bellis MA, Syed Q, An investigation into the microflora of heroin. J Med Microbiol. 2002;51:1001–8 .PubMedGoogle Scholar
- Sapira JD. The narcotic addict as a medical patient. Am J Med. 1968;45:555–88. DOIPubMedGoogle Scholar
- Siegel H, Helpern M, Ehrenreich T. The diagnosis of death from intravenous narcotism. With emphasis on the pathologic aspects. J Forensic Sci. 1966;11:1–16 .PubMedGoogle Scholar
- Werner SB, Passaro D, McGee J, Schechter R, Vugia DJ. Wound botulism in California, 1951–1998: recent epidemic in heroin injectors. Clin Infect Dis. 2000;31:1018–24 DOIPubMedGoogle Scholar
- Hope VD, Palmateer N, Wiessing L, Marongiu A, White J, Ncube F, A decade of spore-forming bacterial infections among European injecting drug users: pronounced regional variation. Am J Public Health. 2012;102:122–5. DOIPubMedGoogle Scholar
- McGuigan CC, Penrice GM, Gruer L, Ahmed S, Goldberg D, Black M, Lethal outbreak of infection with Clostridium novyi type A and other spore-forming organisms in Scottish injecting drug users. J Med Microbiol. 2002;51:971–7 .PubMedGoogle Scholar
- Health Protection Agency. Tetanus: information for health professionals. 2007 [cited 2012 Aug 15]. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947374762
- Health Protection Agency. Wound botulism cases associated with injecting drug use. 2011 [cited 2012 Aug 15]. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Botulism/GeneralInformation/botu020WoundbotulismcasesassociatedwithIDU/
- Taylor A, Hutchinson S, Lingappa J, Wadd S, Ahmed S, Gruer L, Severe illness and death among injecting drug users in Scotland: a case–control study. Epidemiol Infect. 2005;133:193–204. DOIPubMedGoogle Scholar
- Ramsay CN, Stirling A, Smith J, Hawkins G, Brooks T, Hood J, An outbreak of infection with Bacillus anthracis in injecting drug users in Scotland. Euro Surveill. 2010;15:19465 .PubMedGoogle Scholar
- National Anthrax Outbreak Control Team. An outbreak of anthrax among drug users in Scotland, December 2009 to December 2010. Glasgow (UK): Health Protection Scotland, December 2011 [cited 2011 Dec 29]. http://www.documents.hps.scot.nhs.uk/giz/anthrax-outbreak/anthrax-outbreak-report-2011-12.pdf
- Hay G, Gannon M, MacDougall J, Millar T, Eastwood C, McKeganey N. Local and national estimates of the prevalence of opiate use and/or crack cocaine use. In: Singleton N, Murray R, Tinsley L, editors. Measuring different aspects of problem drug use: methodological developments. 2nd ed. London: Home Office; 2006. p. 3–40.
- Hay G, Gannon M, Casey J, McKeganey N. Estimating the national and local prevalence of problem drug misuse in Scotland: executive report. Glasgow (UK): University of Glasgow; 2009 Aug [cited 2011 Dec 15]. http://www.drugmisuse.isdscotland.org/publications/local/Prevalence_2009.pdf
- Hope VD, Judd A, Hickman M, Sutton A, Stimson GV, Parry JV, HIV prevalence among injecting drug users in England and Wales 1990 to 2003: evidence for increased transmission in recent years. AIDS. 2005;19:1207–14 . DOIPubMedGoogle Scholar
- University of the West of Scotland, Health Protection Scotland, West of Scotland Specialist Virology Centre. The Needle Exchange Surveillance Inititative (NESI): prevalence of HCV and injecting risk behaviours among injecting drug users attending needle exchanges in Scotland, 2008/2009. Paisley (UK): University of the West of Scotland; 2010 Apr [cited 2011 Dec 15]. http://www.hepcscotland.co.uk/media/50084/nesi-report-08-09.pdf
- United Nations Office on Drugs and Crime. World drug report 2009. Vienna: The Office; 2009.
- Serious Organised Crime Agency. The United Kingdom threat assessment of organised crime 2009–2010. 2010 [cited 2011 Dec 15]. http://www.soca.gov.uk/about-soca/library?start=10
- Ringertz SH, Hoiby EA, Jensenius M, Maehlen J, Caugant DA, Myklebust A, Injectional anthrax in a heroin skin-popper. Lancet. 2000;356:1574–5. DOIPubMedGoogle Scholar
- Caugrant DA, Fossum K, Hoel T, Høiby EA, Iversen BG, Jensenius M, Systemic anthrax in an injecting drug user: Oslo, Norway, April 2000. Euro Surveill. 2000;4:1605.
- Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Bellis M. Adulterants in illicit drugs: a review of empirical evidence. Drug Test Anal. 2011;3:89–96. DOIPubMedGoogle Scholar
- Horowitz BZ, Swensen E, Marquardt K. Wound botulism associated with black tar heroin. JAMA. 1998;280:1479–80. DOIPubMedGoogle Scholar
- Nicolaier A. On infectious tetanus [in German]. Dtsch Med Wochenschr. 1884;10:842–4. DOIGoogle Scholar
- Peek SF, Semrad SD, Perkins GA. Clostridial myonecrosis in horses (37 cases 1985–2000). Equine Vet J. 2003;35:86–92. DOIPubMedGoogle Scholar
- Hahné SJ, White JM, Crowcroft NS, Brett MM, George RC, Beeching NJ, Tetanus in injecting drug users, United Kingdom. Emerg Infect Dis. 2006;12:709–10. DOIPubMedGoogle Scholar
- Akbulut D, Dennis J, Gent M, Grant KA, Hope V, Ohai C, Wound botulism in injectors of drugs: upsurge in cases in England during 2004. Euro Surveill. 2005;10:172–4 .PubMedGoogle Scholar
- Hankins C, Palmer D, Singh R. Unintended subcutaneous and intramuscular injection by drug users. CMAJ. 2000;163:1425–6 .PubMedGoogle Scholar
- Graham CA, McNaughton GW, Crawford R. ‘Popping’: a cause of soft tissue sepsis in chronic drug abusers. Eur J Emerg Med. 1999;6:259–61. DOIPubMedGoogle Scholar
- Dwyer R, Topp L, Maher L, Power R, Hellard M, Walsh N, Prevalences and correlates of non-viral injecting-related injuries and diseases in a convenience sample of Australian injecting drug users. Drug Alcohol Depend. 2009;100:9–16. DOIPubMedGoogle Scholar
- Hope VD, Marongiu A, Parry JV, Ncube F. The extent of injection site infection in injecting drug users: findings from a national surveillance study. Epidemiol Infect. 2010;138:1510–8. DOIPubMedGoogle Scholar
- English L. Treatment and care for older drug users. In: Eaton D, Davies G, English L, Lodwick A, Bellis M, McVeigh J, editors. National report to the EMCDDA by the Reitox National Focal Point United Kingdom: new developments. London: UK Focal Point; 2009. p. 203–23.
- Health Protection Agency. Anthrax: information on 2010 outbreak. 2011 [cited 2011 Dec 15]. http://www.hpa.org.uk/web/HPAweb&Page&HPAwebAutoListName/Page/1265637163487
- Baymiller S. Botulism in critical care: a case study in wound botulism. Am J Crit Care. 2001;10:172–80 .PubMedGoogle Scholar
- Wenham T, Cohen A. Botulism. Continuing Education in Anaesthesia. Critical Care & Pain. 2008;8:21–5.
- Cook TM, Protheroe RT, Handel JM. Tetanus: a review of the literature. Br J Anaesth. 2001;87:477–87. DOIPubMedGoogle Scholar
- Rushdy AA, White JM, Ramsay ME, Crowcroft NS. Tetanus in England and Wales, 1984–2000. Epidemiol Infect. 2003;130:71–7. DOIPubMedGoogle Scholar
- Hickman M, Higgins V, Hope V, Bellis M, Tilling K, Walker A, Injecting drug use in Brighton, Liverpool, and London: best estimates of prevalence and coverage of public health indicators. J Epidemiol Community Health. 2004;58:766–71. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 19, Number 1—January 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Norah E. Palmateer, Health Protection Scotland, Meridian Court, 5 Cadogan St, Glasgow G2 6QE, Scotland, UK
Top