Volume 19, Number 5—May 2013
Letter
Atypical Erythema Migrans in Patients with PCR-Positive Lyme Disease
To the Editor: The best diagnostic sign in patients with early Lyme disease is a skin lesion, erythema migrans (EM). However this sign may not occur or be recognized in 30% of cases (1). Furthermore, the EM rash may not display a classic bull’s-eye (ring-within-a-ring) appearance, a fact that may be underappreciated (2,3). Some studies noted uncharacteristic variants of EM in 25%–30% of cases (4–7). One study reported the rash to be uniformly red in 60% of cases (6). Other atypical variants of EM are a blue-red appearance and, occasionally, a vesicular central region (4,5). We describe the occurrence of atypical EM in patients with microbiologically proven Borrelia burgdorferi infection.
During spring and summer 2009, a total of 29 patients with classic or possible EM and suspected Lyme disease were referred by primary care physicians for an ongoing prospective study. Laboratory methods have been described (8). The patients were >18 years of age and lived in suburban Baltimore, Maryland, USA, where Lyme disease is endemic. All patients had extracutaneous manifestations (e.g., virus-like symptoms). Fourteen patients met laboratory criteria for study analysis: 1) positive PCR at the initial study visit, detected by a B. burgdorferi–specific nucleic acid–enhancing PCR method on a 1.25-mL whole blood sample (8), and 2) evidence of B. burgdorferi exposure by the 2-tiered antibody test at the initial or posttreatment visit. Other entry criteria were a rash >5 cm and symptoms compatible with early Lyme disease (1); exclusion criteria were certain preexisting medical conditions (8).
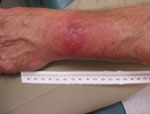
A panel of experienced specialists, including dermatologists, were shown photographs of the patients’ skin lesions and asked if they would expect the average primary care physician to diagnose the lesions as EM. To avoid bias, PCR and serologic test results we withheld from the specialists and they were asked to categorize lesions by characteristics common to target-like and non–target-like lesions. Lesions with the classic bull’s-eye appearance, with central clearing and peripheral erythema, were classified as classic EM; those with uniform red or red-blue or other appearance and lacking central clearing were classified as possible atypical EM. If any lesion of a multiple lesion set was classic in appearance, we categorized the rash as classic EM. Of the 14 patients with positive PCR, 10 had nonclassic EM (Figure) and 4 had classic, target-like EM. Atypical rashes varied from those close to classic EM to those resembling lesions more common in other conditions (e.g., insect or spider bites) and, consequently, prone to misdiagnosis.
Depending on the appearance of an atypical rash, the differential diagnosis could include contact dermatitis, arthropod bite, or, in cases with annular lesions, fixed drug eruptions, granuloma annulare, cellulitis, dermatophytosis, or systemic lupus erythematosus (5). In addition, a diagnosis can be more challenging when there are multiple skin lesions rather than a single lesion and in a pattern unfamiliar to a general practitioner.
Multiple textbooks and websites have featured pictures of EM as a bull’s-eye lesion (Technical Appendix). This emphasis on target-like lesions may have inadvertently contributed to an underappreciation for atypical skin lesions caused by Lyme disease. Nevertheless, physician recognition of Lyme disease–associated EM is essential because current approved laboratory tests may not identify B. burgdorferi in the first few weeks of infection (8), when an accurate diagnosis can lead to early curative therapy.
Separate studies found different percentages of atypical Lyme disease–associated rashes (3,4,9); each was lower than the percentage found in our study. Our study has several limitations: it encompassed only 1 recruitment season, 1 geographic site, and a small number of patients. The sensitivity of PCR for blood specimens is improving (8); however, PCR may have missed some acute cases in our study for reasons cited below. Therefore, these patients should not obligatorily be considered as representative of all acute Lyme disease patients.
Our study results serve as an impetus for studying more patients with systemic and nonsystemic signs and symptoms over multiple seasons and geographic areas and for including PCR analysis of skin lesions in future studies. PCR of skin biopsy samples may provide insight as to whether a negative blood PCR is the result of infection with a skin-restricted strain (10) in patients in whom bacterial dissemination is not expected or a result of low copy number of B. burgdorferi in the blood sample.
Our results serve as a reminder that patients with early Lyme disease may have an atypical rash, not the classic (textbook) bull’s-eye lesion. Close observation and a detailed history of whether the rash is enlarging, has enlarged, or is spreading should be part of the consideration of the diagnosis. Observation for extracutaneous signs of early infection, such as cranial seventh nerve palsy (Bell’s palsy) or meningitis, is also essential.
In summary, the EM rash of Lyme disease can have an atypical appearance. Thus, clinicians should consider Lyme disease in the differential diagnosis of patients who have a rash that may not be classic EM and who have been in areas where Lyme disease occurs.
Acknowledgment
Funding for this study was provided by a grant (AI077156) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- Centers for Disease Control and Prevention. Lyme disease [cited 2012 Oct 23]. http://www.cdc.gov/lyme
- Feder HM Jr, Whitaker DL. Misdiagnosis of erythema migrans. Am J Med. 1995;99:412–9. DOIGoogle Scholar
- Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–15. DOIGoogle Scholar
- Berger BW, Johnson RC, Kodner C, Coleman L. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J Clin Microbiol. 1992;30:359–61 .
- Smith RP, Schoen RT, Rahn DW, Sikand VK, Nowakowski J, Parenti DL, Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. 2002;136:421–8 . DOIGoogle Scholar
- Nadelman RB, Wormser GP. Recognition and treatment of erythema migrans: are we off target? Ann Intern Med. 2002;136:477–9 . DOIGoogle Scholar
- Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS ONE. 2012;7:e36825. DOIGoogle Scholar
- Berger BW, Lesser RL. Lyme disease. Dermatol Clin. 1992;10:763–75 .
- Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–24 .
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 19, Number 5—May 2013
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Steven E. Schutzer, University of Medicine and Dentistry New Jersey Medical School, Newark, NJ 07103, USA
Top