Volume 19, Number 8—August 2013
Dispatch
Macrolide Resistance of Mycoplasma pneumoniae, South Korea, 2000–2011
Abstract
In Korea, Mycoplasma pneumoniae was detected in 255/2,089 respiratory specimens collected during 2000–2011; 80 isolates carried 23S rRNA gene mutations, and 69/123 culture-positive samples with the mutation were resistant to 5 macrolides. During 2000–2011, prevalence of the mutation increased substantially. These findings have critical implications for the treatment of children with mycoplasma pneumonia.
Mycoplasma pneumoniae is 1 of the most common causes of community-acquired pneumonia in children and young adults (1). Epidemics of mycoplasma pneumonia typically occur every 4–7 years; however, epidemics have occurred every 3–4 years in South Korea (2,3). The first-line treatment for mycoplasma pneumonia is macrolide antimicrobial drugs, but macrolide-resistant infections have been recognized in conjunction with an increase in cases in children in Japan, China, Germany, France, Israel, and the United States (1,4–10). Because of the risk to children administered tetracycline and fluoroquinolone (nonmacrolide drugs), M. pneumoniae resistance to macrolide drugs has critical implications for the treatment of mycoplasma pneumonia in children. This study was conducted to identify the prevalence of macrolide resistance among M. pneumoniae strains isolated from children with lower respiratory tract infections (LRTIs) during 4 consecutive epidemics (2000–2011) in South Korea.
A total of 2,089 respiratory samples were tested for the presence of M. pneumoniae. Of these, a total of 378 were archived samples collected during epidemics in 2000 (71 samples), 2003 (112 samples), and 2006 (195 samples), and 1,711 were samples collected and tested during August 2010–December 2011. Specimens from the 2010–2011 epidemic were collected from children at Seoul National University Children’s Hospital, Seoul National University Bundang Hospital, and Seoul Eulji Hospital. All samples were obtained from children (median age 5 years, range 6 months–18 years) with a diagnosis of community-acquired LRTI.
P1 adhesin was amplified by PCR for the detection of M. pneumoniae from the 378 archived samples. M. pneumoniae was cultivated by using pleuropneumonia-like organism broth and agar for the 1,711 samples collected during 2010–2011. Media were incubated aerobically at 37°C for 6 weeks. Plates were observed daily to identify change in the color of the broth from red to transparent orange. When the color changed, the samples were subcultured on agar plates. Spherical M. pneumoniae colonies were observed by using a microscope.
For the cultured M. pneumoniae isolates, we amplified domain V of the 23S rRNA gene by PCR; for the archived samples, we extracted DNA. For PCR, we used primers MP23SV-F 5′-TAACTATAACGGTCCTAAGG-3′ and MP23SV-R 5′-ACACTTAGATGCTTTCAGCG-3′. The PCR products were sequenced to identify mutations. Sixty-four of the M. pneumoniae–positive samples from 2000 and 2003 had been previously tested for mutations in the 23S rRNA gene.
Minimum inhibitory concentrations (MICs) were measured by using the microdilution method in triplicate for the following antimicrobial agents: erythromycin, clarithromycin, azithromycin, roxithromycin, josamycin, tetracycline, doxycycline, levofloxacin, moxifloxacin, and ciprofloxacin. MIC was defined as the lowest antimicrobial drug concentration at which the media color did not change at the time when the color of the positive control media (containing M. pneumoniae strains only) changed (11).
M. pneumoniae was detected in 255 (12.2%) of 2,089 clinical samples; 132 (51.8%) of the positive samples were among the 378 archived samples, and 123 (48.2%) were among the 1,711 samples from 2010–2011. For the 132 archived samples, M. pneumoniae was detected by PCR: 30 (22.8%) were among the 71 samples from 2000, 34 (25.8% were among the 112 samples from 2003, and 68 (51.5%) were among the 195 samples from 2006. For the 123 samples from 2010–2011, M. pneumoniae was detected by culture.
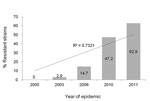
Overall, 80 (31.4%) of the 255 M. pneumoniae–positive samples carried mutations in the 23S rRNA gene. Of these, 78 had the A2063G transition and 2 exhibited the A2064G transition. The prevalence of the 23S rRNA mutation increased significantly over the 4 consecutive epidemics, as follows: 2000 epidemic, 0 of 30 samples; 2003 epidemic, 1 (2.9%) of 34 samples; 2006 epidemic, 10 (14.7%) of 68 samples; and 2010–2011 epidemic, 25 (47.2%) of 53 samples in 2010 and 44 (62.9%) of 70 samples in 2011 (p<0.001 for trend) (Figure).
Among 123 samples culture-positive for M. pneumoniae, 69 that carried the 23S rRNA mutation exhibited significantly higher MIC50 (MIC for 50% of strains) and MIC90 when tested with 5 macrolides, compared with 54 strains that lacked the mutation. For example, the MIC50 and MIC90 of erythromycin were 16 µg/mL and 128 µg/mL, respectively, for strains with the 23S rRNA mutation and 0.001 µg/mL and 0.002 µg/mL, respectively, for strains without the mutation (p<0.0001) (Table 1). All 123 M. pneumoniae strains were susceptible to nonmacrolide antimicrobial drugs, including tetracycline, doxycycline, levofloxacin, ciprofloxacin, and moxifloxacin (Table 2).
Macrolide resistance is associated with point mutations in domain V of the M. pneumoniae 23S rRNA gene, especially those corresponding to A2063G or A2064G transitions (4,5,7). Thus, emergence of macrolide-resistant strains may result in treatment failure of M. pneumoniae infections (4).
We did not detect macrolide resistance among M. pneumoniae strains collected during 2000; thereafter, the prevalence of macrolide resistance remained low through the 2003 epidemic. Macrolide resistance then increased to 14.7% during the epidemic of 2006 and to 56.1% during the epidemic of 2010–2011, as indicated by substantially higher MICs against macrolide agents in association with the presence of the 23S rRNA gene mutation in M. pneumoniae isolates.
Macrolide resistance has been detected with increasing frequency in many parts of the world, highlighting the importance of knowing the geographic distribution and temporal patterns of macrolide-resistant M. pneumoniae. After the first isolation of a macrolide-resistant strain in 2001, Japan reported a dramatic increase in macrolide resistance among children with mycoplasma pneumonia, and in 2011 resistance was >80% (12,13). China identified an 83%–92% prevalence of macrolide-resistant M. pneumoniae isolates (6,9). In contrast, France identified only 2 resistant M. pneumoniae isolates during 1994–2006, and the United States reported a 30% prevalence of macrolide-resistant strains (10). Israel reported that in 2010, ≈30% of M. pneumoniae isolates carried an A2063G transition in domain V of the 23S rRNA gene (8). In Italy, 26% of M. pneumoniae–infected children harbored strains with point mutations in domain V of the 23S rRNA gene (14). Thus, there is great variability in the prevalence of macrolide resistance in M. pneumoniae isolates.
A key finding of this study is the increasing prevalence of macrolide resistance over time. Several factors may have led to this increase. First, the increased use of macrolide antimicrobial drugs may be responsible for the development and spread of macrolide resistance. A recent study showed a correlation between increased use of oral macrolides and an increase in macrolide resistance by selective pressure in M. pneumoniae and other respiratory pathogens (13). A comprehensive trend analysis of the national data showed an increase in macrolide use in the community (expressed in defined daily doses [DDD]/1,000 inhabitants/day) during 2005–2009 (15). Penicillins and cephalosporins are the 2 most frequently used classes of oral antimicrobial drugs. There were decreasing trends in penicillin use and a subtle increase in cephalosporin use during 2005–2009. Macrolide use remained steady until 2007; however, there was an increase of >30% in DDD/1,000 inhabitants/day between 2007 (2.5 DDD/1,000 inhabitants/day) and 2009 (3.3 DDD/1,000 inhabitants/day). Our data on macrolide use do not fully explain the 10-year change in M. pneumoniae resistance to macrolide drugs because data were available only for 2005–2009. In addition, the spread of resistant strains could have been facilitated by other factors, such as high population density or geographic closeness with the 2 neighboring countries, where resistant strains were highly prevalent.
We found an increasing prevalence of the 23S rRNA gene mutation in M. pneumoniae isolates during 2000–2011 in South Korea. We did not address the clinical issues regarding antimicrobial drug choices for macrolide-resistant mycoplasma pneumonia or compare the clinical outcomes for macrolide-resistant and macrolide-sensitive infections; nevertheless, we believe that the evidence of a recent increase in macrolide resistance provides guidance for additional clinical investigations and new therapeutic strategies. The incidences of macrolide-resistant M. pneumoniae infection should be carefully monitored, particularly among children, for whom treatment can be challenging. Further studies are needed to evaluate the clinical significance of macrolide-resistant M. pneumoniae pneumonia.
Dr Hong is a clinical fellow in pediatric infectious diseases at Seoul National University Hospital and has a special interest in childhood pneumonia.
Acknowledgments
This work was presented at the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy in San Francisco, California, USA, September 9–12, 2012.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, which is funded by the Ministry of Education, Science and Technology (2010-0024874).
References
- Matsuoka M, Narita M, Okazaki N, Ohya H, Yamazaki T, Ouchi K, Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob Agents Chemother. 2004;48:4624–30. DOIPubMedGoogle Scholar
- Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32:956–73. DOIPubMedGoogle Scholar
- Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect. 2008;56:326–31. DOIPubMedGoogle Scholar
- Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother. 2008;52:348–50. DOIPubMedGoogle Scholar
- Peuchant O, Menard A, Renaudin H, Morozumi M, Ubukata K, Bebear CM, Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J Antimicrob Chemother. 2009;64:52–8. DOIPubMedGoogle Scholar
- Xin D, Mi Z, Han X, Qin L, Li J, Wei T, Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrob Agents Chemother. 2009;53:2158–9. DOIPubMedGoogle Scholar
- Dumke R, von Baum H, Luck PC, Jacobs E. Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clin Microbiol Infect. 2010;16:613–6. DOIPubMedGoogle Scholar
- Averbuch D, Hidalgo-Grass C, Moses AE, Engelhard D, Nir-Paz R. Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerg Infect Dis. 2011;17:1079–82. DOIPubMedGoogle Scholar
- Liu Y, Ye X, Zhang H, Xu X, Li W, Zhu D, Antimicrobial susceptibility of Mycoplasma pneumoniae isolates and molecular analysis of macrolide-resistant strains from Shanghai, China. Antimicrob Agents Chemother. 2009;53:2160–2. DOIPubMedGoogle Scholar
- Wolff BJ, Thacker WL, Schwartz SB, Winchell JM. Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high-resolution melt analysis. Antimicrob Agents Chemother. 2008;52:3542–9. DOIPubMedGoogle Scholar
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing—twenty-first information supplement: approved standard M100-S21. Wayne (PA): The Institute; 2011.
- Okazaki N, Narita M, Yamada S, Izumikawa K, Umetsu M, Kenri T, Characteristics of macrolide-resistant Mycoplasma pneumoniae strains isolated from patients and induced with erythromycin in vitro. Microbiol Immunol. 2001;45:617–20 .PubMedGoogle Scholar
- Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012;55:1642–9. DOIPubMedGoogle Scholar
- Chironna M, Sallustio A, Esposito S, Perulli M, Chinellato I, Di Bari C, Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J Antimicrob Chemother. 2011;66:734–7. DOIPubMedGoogle Scholar
- Korean Centers for Disease Control and Prevention, Korea National Institute of Health. Korean Antimicrobial Resistance Monitoring System annual report. Chungbuk (South Korea) The Centers; 2011.
Figure
Tables
Cite This ArticleTable of Contents – Volume 19, Number 8—August 2013
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondence: Eun Hwa Choi, Seoul National University Children's Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 110-769, South Korea
Top