Volume 20, Number 6—June 2014
Letter
Genetic Relatedness of Dolphin Rhabdovirus with Fish Rhabdoviruses
To the Editor: Rhabdoviruses are enveloped, single-stranded, negative-sense RNA viruses that comprise a large and diverse family in the order Mononegavirales and infect arthropods, plants, fish, and mammals. There are 9 genera of rhabdoviruses: Cytorhabdovirus, Ephemerovirus, Lyssavirus, Novirhabdovirus, Nucleorhabdovirus, Perhabdovirus, Sigmavirus, Tibrovirus, and Vesiculovirus. In addition, a substantial number of plant, vertebrate, and invertebrate rhabdoviruses have not been classified (1). Three genera (Novirhabdovirus, Perhabdovirus, and Vesiculovirus) comprise members that infect fresh water and marine fish (2). Fish rhabdoviruses pose a serious problem for aquaculture because of worldwide outbreaks of disease caused by novirhabdoviruses, perhabdoviruses, and vesiculoviruses (3,4).
In 1992, a rhabdovirus-like virus was isolated from lung and kidney of a white-beaked dolphin (Lagenorhynchus albirostris) that had beached along the coast of the Netherlands (5). Although no macroscopic or microscopic lesions were observed at necropsy, negative contrast electron microscopy showed typical rhabdovirus-like, bullet-shaped particles in Vero cell cultures that showed a focal cytopathic effect (5). After this rhabdovirus-like virus was injected intracerebrally into brains of 1-day-old suckling mice, they died within 5 days (5). We report genetic and phylogenetic characterization of a dolphin rhabdovirus (DRV) and evaluated the seroprevalence of DRV-neutralizing antibodies by using serum samples from various marine mammals collected during a 10-year period (2003–2012).
To characterize DRV, we performed random sequence amplification and deep sequencing with the 454 GS Junior Instrument (Roche, Basel, Switzerland) with DRV-infected Vero cell supernatants as described (6). From this analysis, we determined the complete coding sequence of DRV covered by 42,080 of 49,292 reads (minimum coverage 4 reads, average coverage 872 reads).
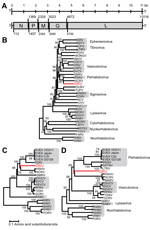
Genomic termini of DRV were determined by using a 3′ and 5′ rapid amplification of cDNA ends PCR and Sanger sequencing of obtained PCR amplicons. The complete genome of DRV (GenBank accession no. KF958252) consists of 11,141 nt and has a typical rhabdovirus gene arrangement of 5 major open reading frames (ORFs) in the order 3′-nucleoprotein (N), phosphoprotein (P), matrix (M) protein, glycoprotein (G), and large (L) protein-5′ (Figure, panel A, Appendix). No additional ORFs ≥300 nt were detected. Between the major ORFs of DRV, intergenic sequences were present that ranged in size from 34 (P–M) to 83 (G–L) nucleotides. Putative transcription initiation and transcription termination polyadenylation sequences were AACA(G/U) and AUGA7, respectively.
The deduced amino acid sequence of genes of DRV and several other rhabdoviruses were aligned by using MUSCLE in MEGA5 version 5.2) (7). Ambiguous aligned regions were removed by using the Gblocks program (8). Phylogenetic analysis of the L and G genes was performed by using the neighbor-joining method in MEGA5 (7). This analysis showed that DRV is most closely related to fish rhabdoviruses of the genera Perhabdovirus and Vesiculovirus and unassigned fish rhabdoviruses with strong bootstrap support (Figure, panels B–D, Appendix).
Deduced amino acid sequences of the 5 major genes had the highest, although weak, homology with those of various fish rhabdoviruses by pairwise identity analyses: N (48%) with hybrid snakehead virus (HSHV), Monopterus albus rhabdovirus (MARV), and Siniperca chautsi rhabdovirus (SCRV); P (18%–20%) with eel virus European X (EVEX), HSHV, MARV, and SCRV; M (27%–33%) with lake trout rhabdovirus, Swedish sea trout rhabdovirus, and EVEX; G (30%–32%) with perch rhabdovirus, lake trout rhabdovirus, Swedish sea trout rhabdovirus, HSHV, MARV, SCRV, and EVEX; and L (54%–56%) with perch rhabdovirus, HSHV, and EVEX. This close relationship with fish rhabdoviruses is surprising because DRV was isolated from tissues of a mammal and propagated in mammalian cell lines at 37°C, which does not occur with related viruses isolated from fish.
To evaluate whether DRV or related viruses circulate among species of cetaceans, we performed serologic screening by using a virus neutralization assay as described (5). The specificity of this assay was tested by using a panel of rhabdovirus-specific antisera obtained from cetaceans of various species (5). The serum samples had been collected for diagnostic purposes from mainly juvenile cetaceans stranded along the coast of the Netherlands during 2003–2012. These species included 2 Atlantic white-sided dolphins (Lagenorhynchus acutus), 79 harbor porpoises (Phocoena phocoena), 9 striped dolphins (Stenella coeruleoabla), and 6 white-beaked dolphins (Lagenorhynchus aIbirostris). Serum samples from 145 bottlenose dolphins (Tursiops truncates) from the collection of the Dolphinarium Harderwijk (Harderwijk, the Netherlands) were also tested. DRV-neutralizing antibodies were detected in serum samples from 1 bottlenose dolphin (7%), 5 striped dolphins (55%), 1 white-beaked dolphin (17%), and 3 harbor porpoises (4%). These results suggested that DRV or closely related viruses continue to infect members of cetacean species (6).
Although rhabdovirus evolutionary pathways are complicated (9), our analysis suggests that DRV is a possible derivative of fish rhabdoviruses. DRV might have originated from an unidentified fish rhabdovirus and might cycle between fish and marine mammals, similar to that suggested for cycling of vesicular stomatitis virus between arthropods and terrestrial mammals (10). Future analyses of sequences from other marine mammal rhabdovirus sequences might support the validity of our phylogenetic analysis and result in creation of a new group containing marine mammal rhabdoviruses.
Acknowledgment
This study was supported by the European Community Seventh Framework Program (FP7/2007–2013) under the project European Management Platform for Emerging and Re-emerging Infectious Disease Entities, European Community grant agreement no. 223498, and by the Virgo Consortium.
References
- King AM, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. London; Waltham (MA): Academic Press; 2012.
- Hoffmann B, Beer M, Schütze H, Mettenleiter TC. Fish rhabdoviruses: molecular epidemiology and evolution. Curr Top Microbiol Immunol. 2005;292:81–117. DOIPubMedGoogle Scholar
- Crane M, Hyatt A. Viruses of fish: an overview of significant pathogens. Viruses. 2011;3:2025–46. DOIPubMedGoogle Scholar
- van Beurden SJ, Engelsma MY, Roozenburg I, Voorbergen-Laarman MA, van Tulden PW, Kerkhoff S, Viral diseases of wild and farmed European eel Anguilla anguilla with particular reference to the Netherlands. Dis Aquat Organ. 2012;101:69–86. DOIPubMedGoogle Scholar
- Osterhaus AD, Broeders HW, Teppema JS, Kuiken T, House JA, Vos HW, Isolation of a virus with rhabdovirus morphology from a white-beaked dolphin (Lagenorhynchus albirostris). Arch Virol. 1993;133:189–93. DOIPubMedGoogle Scholar
- van Leeuwen M, Williams MM, Koraka P, Simon JH, Smits SL, Osterhaus AD. Human picobirnaviruses identified by molecular screening of diarrhea samples. J Clin Microbiol. 2010;48:1787–94. DOIPubMedGoogle Scholar
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. DOIPubMedGoogle Scholar
- Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56:564–77. DOIPubMedGoogle Scholar
- Kuzmin IV, Novella IS, Dietzgen RG, Padhi A, Rupprecht CE. The rhabdoviruses: biodiversity, phylogenetics, and evolution. Infect Genet Evol. 2009;9:541–53. DOIPubMedGoogle Scholar
- Novella IS, Ebendick-Corpus BE, Zárate S, Miller EL. Emergence of mammalian cell-adapted vesicular stomatitis virus from persistent infections of insect vector cells. J Virol. 2007;81:6664–8 . DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 20, Number 6—June 2014
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Rogier Bodewes, Department of Viroscience, Erasmus Medical Centre, Dr. Molewaterplein 50, 3015GE Rotterdam, the Netherlands
Top