Volume 12, Number 10—October 2006
Perspective
Birds and Influenza H5N1 Virus Movement to and within North America
Avian Influenza in Humans
Birds as HPAI H5N1 Reservoirs and Introductory Hosts in the Old World
Possible Role of Birds in Arrival of HPAI H5N1 Avian Influenza in New World
Possible Role of Birds in Movement of HPAI H5N1 in Western Hemisphere
Conclusions
Cite This Article
Abstract
Highly pathogenic avian influenza (HPAI) H5N1 expanded considerably during 2005 and early 2006 in both avian host species and geographic distribution. Domestic waterfowl and migratory birds are reservoirs, but lethality of this subtype appeared to initially limit migrant effectiveness as introductory hosts. This situation may have changed, as HPAI H5N1 has recently expanded across Eurasia and into Europe and Africa. Birds could introduce HPAI H5N1 to the Western Hemisphere through migration, vagrancy, and importation by people. Vagrants and migratory birds are not likely interhemispheric introductory hosts; import of infected domestic or pet birds is more probable. If reassortment or mutation were to produce a virus adapted for rapid transmission among humans, birds would be unlikely introductory hosts because of differences in viral transmission mechanisms among major host groups (i.e., gastrointestinal for birds, respiratory for humans). Another possible result of reassortment would be a less lethal form of avian influenza, more readily spread by birds.
Avian influenza virus A refers collectively to a group of viruses within the family Orthomyxoviridae that has a worldwide distribution and causes a variety of diseases in birds. Classification of influenza viruses is based on 2 glycoproteins (antigens) characteristic of the group members: hemagglutinin, of which 16 forms are known; and neuraminidase, of which 9 forms have been described. In 1997, a virulent, highly pathogenic avian influenza (HPAI) A virus, identified as the H5N1 subtype, was identified in samples taken in Hong Kong (1,2). This virus has spread to several localities in Asia and, since late 2005, Europe (3) and Africa (4) (Table 1). HPAI H5N1 virus is found most commonly in domestic fowl, although as of late 2005, it has been found in migratory and resident birds of several orders (mainly Anseriformes) and in pigs, civets, house cats, tigers, leopards, and humans (3). This virus poses a potential danger to human populations; 224 human cases of H5N1 avian influenza have been reported as of May 29, 2006; 127 of these cases were fatal (17). Its discovery in migratory birds is especially troubling because of the potential for rapid dispersal of the virus across continents and hemispheres.
We review facts concerning outbreaks of H5N1; the species of birds, especially migrants, known to have been infected by this subtype; and available information on the ability of migrants to serve as reservoir or introductory hosts that move the virus from outbreak areas to new localities. On the basis of this information, we consider the avian pathways by which HPAI H5N1 might enter the Western Hemisphere and, once present, the likelihood that it will be able to disperse to new regions. We define migratory or migrant birds as those species that move annually between geographically separate breeding and wintering quarters. Migrating birds are those actually in the process of moving from 1 locality to another.
Avian influenza A viruses are common and widespread in birds. Most viruses in this family attack the intestinal tract of the host preferentially and are spread mainly by shedding in host feces (18,19). Waterfowl, e.g., ducks, geese, and swans (Anseriformes), and shorebirds (Charadriiformes) are particularly susceptible because they are exposed to water that may be contaminated with infected fecal matter, especially at specific sites and seasons, when these birds congregate densely at relatively confined and shallow water bodies (Figure 1). A secondary mode of viral spread is consumption of infected avian host parts by predators, including captive carnivores, avian raptors, and carrion-feeding vertebrates. Infection by most avian influenza A strains appears to be asymptomatic for the host (18). Proportions of birds shedding active virus can be high (e.g., >30% in some Canadian duck populations) among juvenile waterfowl gathered in large flocks on lakes and ponds during the summer postbreeding molting period but decrease rapidly during southward migration, falling to 1% to 2% during winter (18). Nevertheless, shedding of active virus can remain as high as 0.25% by individual birds among northbound spring migrants, sufficient to reinfect northern breeding populations (18).
Most birds appear to be more or less susceptible to >1 strain of avian influenza A, but rates of infection and levels of susceptibility to the different viral subtypes vary among taxa. For instance, H3 and H6 subtypes are common in ducks, geese, and swans (Anseriformes), while H4, H9, H11, and H13 subtypes are more prevalent in sandpipers, terns, and gulls (Charadriiformes) (20). The best opportunities for viral transmission among large numbers of anseriform hosts would likely be on lakes and ponds in summer, where large concentrations gather for weeks to undergo the postbreeding, premigratory molt (18). For charadriiformes, the greatest viral transmission opportunities would likely be at stopover sites during fall migration, where tens of thousands of individual birds congregate to feed and roost (20).
Humans and other mammals normally are not susceptible to infection by avian influenza A viruses. Nevertheless, several subtypes of avian influenza or bird-origin influenza viruses have infected humans; 3 of these subtypes have caused pandemics within the past century. At present, HPAI H5N1 is entirely an avian influenza subtype. Humans can become infected, but so far as is known, they must inhale or ingest massive viral doses from excreta or tissues of infected birds to do so. Although clinically ill humans have high death rates, ≈50%, passage of H5N1 virus from human to human is rare (3).
The more humans infected with HPAI H5N1, the greater the probability that reassortment with a human influenza virus strain will occur and produce a lethal form that is spread readily between humans (18,19). However, viral interhost transmission strategies differ fundamentally for those viruses that primarily infect humans versus those that infect birds. Bird viruses have an affinity for the host's intestinal tract, and interhost transmission occurs mainly by fecal contamination of shared water bodies. Human viruses more often attack the respiratory system and depend on shedding in respiratory effluvia for interhost transfer. If, or when, a reassortment or mutation of HPAI H5N1 produces a virus capable of efficient horizontal transfer among humans, the new virus would likely not be particularly effective in transfer among birds; migrants likely would play little role in spread of such a virus. Vaccines produced to prevent human infection by H5N1 might not be effective against a new virus produced by reassortment.
The main reservoirs and introductory hosts for avian influenza A viruses in general are migratory waterfowl and domestic fowl (18,19). HPAI H5N1, however, causes high rates of disabling illness and death in most avian species (21). High rates of illness would prevent migrants from being introductory hosts, since sick wild birds normally cannot move far and do not survive long. Thus, perhaps not surprisingly, no evidence exists that migrants were introductory hosts for H5N1 for several years after its initial appearance in Guangdong Province, People's Republic of China, in 1996. In fact, no deaths or even infections of migrants were reported until December 2002, when several migrants and exotic birds were found dead at a Hong Kong park and zoologic garden (10). Of 3,095 outbreaks of HPAI H5N1 reported from December 2003 through February 2005, all involved captive birds or domestic fowl (6). Until early August 2005, only 2 outbreaks of HPAI H5N1 had been confirmed in migratory birds presumed to be completely separate from domestic fowl: Qinghai Lake and Xinjiang Province, China, (April, May 2005) (12) and Lakes Erhel and Khunt in northern Mongolia (August 2005) (15). However, that situation has changed, and several new outbreaks have been recorded in migrants that were presumably separate from domestic fowl within the last few months (Appendix Table), perhaps signaling genetic modification of the virus (19).
Data based on observations of dead wild birds at sites where infections have broken out and negative results from subsequent extensive screening for seropositive or infected migrants around outbreak sites have indicated that HPAI H5N1 was lethal for most wild birds, at least until recently. Nevertheless, some studies have demonstrated that chickens, domestic ducks, and geese infected under laboratory conditions, as well as some wild birds exposed under quasilaboratory conditions (e.g., birds fed, watered, and protected at zoologic parks or gardens), survive infection and shed the virus in active form (10,22,23). The work by Komar et al. (24) on wild birds exposed to West Nile virus (WNV) under laboratory conditions may be instructive in this regard. These researchers found that in species like the fish crow (Corvus ossifragus), in which individual birds were known to have high death rates on exposure to the New York 99 subtype of WNV in the wild (on the basis of large numbers of birds found dead and failure to find free-flying birds captured that were seropositive), survival rates from exposure in the laboratory were 45%. When one considers that birds kept in a laboratory have ready access to food and water during their illness, as well as protection from inclement weather and predators, this finding perhaps is not surprising. However, wild birds associating with free-ranging domestic fowl at farm ponds, or captive exotic birds at city parks or zoological gardens, may receive some of the same benefits as laboratory birds, experiencing conditions conducive to survival of infection by HPAI H5N1.
Recent detections of HPAI H5N1 in free-ranging migrants may be a result of heightened awareness and thus the virus could have been circulating in migrants, although undetected. This explanation is unlikely considering the extensive screening of blood and feces of migrants in the past several years in Europe, parts of Asia, and North America. These screenings have searched for birds seropositive for H5N1 and other avian influenza type A viruses. These searches have involved sampling thousands of birds of hundreds of species (25,26). The virus may also have changed to some degree (2,19), allowing higher survival rates among some species of migrants. Both explanations may have some relevance to the current situation. In any event, some migratory birds may now be able to move HPAI H5N1 in active form over considerable distances (Table A1). Increasing numbers of recent reports document apparent movement of the virus, whereas before April 2005, no evidence existed of HPAI H5N1 in free-ranging migratory birds distant from domestic fowl, despite years of sampling of tens of thousands of migratory waterfowl of several species from wetland sites across the European continent (25).
To date, HPAI H5N1 has not been recorded in the New World, although outbreaks of related avian influenza viruses lethal to domestic fowl have occurred in Ontario, Canada, in 1966 (H5N9); Pennsylvania, United States in 1983 (H5N2); Puebla, Mexico, in 1994 (H5N2); Chile in 2002 (H7N3); Canada in 2004 (H7N3); and Texas, United States, in 2004 (H5N2) (27). All of these outbreaks occurred in domestic poultry and were controlled without further diffusion. We see 3 possible modes by which HPAI H5N1 might gain entry to the New World if birds were the introductory host: 1) normal interhemispheric migration, 2) vagrancy, and 3) legal and illegal importation of birds as explained in the following section.
Normal Interhemispheric Migration
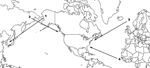
Few individual birds within few species undertake regular, interhemispheric migration. However, some do, and the waterfowl (Anseriformes, Charadriiformes, Ciconiiformes) could be introductory hosts for HPAI H5N1 to the New World (Table 2). Three pathways are used annually by a small number of waterfowl species to travel between the hemispheres: 1) Alaska–East Asia, in which birds that breed in Alaska winter in East Asia; 2) East Asia–Pacific North America, in which birds that breed in northeast Asia winter along the Pacific Coast of North America; and 3) Europe–Atlantic North America, in which birds that breed in Iceland or northwestern Europe winter along the Atlantic Coast of North America (Figure 2, Table 2).
Two lines of evidence argue against normal, interhemispheric migration as a likely mode of entry for HPAI H5N1 into the Western Hemisphere. First, as discussed previously, data indicate that most infected individual birds of most species of migrants become extremely ill and either cannot migrate far in their weakened state or die at the place of infection. Second, investigation of the genetics of avian influenza viruses has shown that little natural interchange occurs between the Eastern and Western Hemispheres: each hemisphere appears to have an avian influenza virus community that is largely distinct (18). This fact is particularly noteworthy when one considers that most avian influenza A viruses appear to be asymptomatic, and migrants readily transport them in infectious form, in stark contrast to the situation for HPAI H5N1. Presumably, the distinct nature of the avian influenza A community in each hemisphere results from the fact that the main reservoir for these viruses is migrants, and few migrants move regularly between the hemispheres (32).
Vagrancy
Perhaps a third or more of Eurasian waterfowl species have traveled into the Western Hemisphere as vagrants; some occur more regularly than others, including those listed in Table 2. However, all Eurasian vagrants are, by definition, extremely rare in the New World (a few birds per decade). One mode of interhemispheric vagrancy is tropical storm systems that originate off the West African coast during the Atlantic hurricane season, which lasts from June to November each year. These systems can, and occasionally do, sweep up and transport Old World birds, especially waterfowl, across the Atlantic to the New World (route 4, Figure 2). Vagrancy is much rarer (by several orders of magnitude) than normal interhemispheric migration and seems an even less likely mode of entry for HPAI H5N1.
Legal and Illegal Importations
Human traffic in birds and bird products is the sole documented means of HPAI H5N1 movement between geographically separate regions to date (19). While migratory birds have been suspected of involvement, particularly in cases in which no obvious human interchange of infected birds or products has occurred, these conclusions are inferred (19). Thus, if HPAI H5N1 is to be kept out of the Western Hemisphere, control of legal and illegal imports should be the primary focus of prevention efforts.
The legal importation of exotic birds has declined dramatically in the United States since enactment of the 1992 Wild Bird Conservation Act. Nevertheless, 2,770 birds entered the country through the New York port of entry in 1999, including 323 pet birds and 2,447 commercial birds. In addition, 12,931 birds passed through in transit (S. Kaman, US Department of Agriculture [USDA], pers. comm.) Legal importations are controlled by USDA Animal and Plant Health Inspection Service and the US Fish and Wildlife Service. Most imported birds undergo a 30-day quarantine at USDA facilities located near each of the 3 allowed ports of entry: New York, Miami, and Los Angeles. Quarantine procedures include isolation in indoor, air-filtered cages and standard testing for common poultry diseases, including avian influenza. The number of illegally imported birds is not known. These birds are not subject to quarantine and testing and could be a mode of entry for HPAI H5N1. Hawk eagles from Thailand infected with the virus were recently detected while being smuggled into Belgium (11). Although these birds were detected and quarantined, they serve as an example of how such imports could spread the virus. Species commonly associated with the transhemispheric bird trade are listed in Table 2.
If birds turn out to be responsible for entry of HPAI H5N1 into the Western Hemisphere, illegal import of an infected bird or bird product seems the most likely mode of entry. We base this conclusion on the fact that illegally imported birds, unlike infected, free-flying migrants, are provided food and water ad libitum and protected from predators, greatly increasing their chances of survival in an infectious state. Furthermore, these birds often end up in close association with other, similarly protected birds, sharing the same food or water, a situation that provides ample opportunity for viral transmission.
Movement of HPAI H5N1 by sale of infected domestic fowl or poultry products in the United States and Canada is unlikely, given existing regulations. Thus, a major mode of HPAI spread available in much of Eurasia would be ruled out. Also, most domestic fowl are kept separate from wild migratory waterfowl in both countries, which would rule out a second major mode of introduction and cross-infection. Mixing of wild migratory birds with captive, exotic birds is relatively common, however, at North American zoos. Birds in such exhibits should be screened regularly for H5N1 or whatever HPAI virus is in circulation during a given year.
The HPAI H5N1 subtype of avian influenza A causes high mortality rates in most wild birds, at least in its present form. The situation is similar to that found for the form of WNV introduced into the Western Hemisphere in 1999 (24,32–34). Even under conditions in which food, water, and protection from predators are provided, death rates are high. These kinds of death rates could result if the current form of HPAI H5N1 were introduced into New World bird populations. In such a scenario, migrants might not be capable of moving the virus far from its point of introduction, at least initially. Also, the die-offs occurring at the site of entry likely would be obvious to wildlife disease monitors, which would allow for rapid quarantine. However, if the H5N1 virus were introduced into the Western Hemisphere, migratory birds, particularly anseriforms (ducks, swans, geese), might serve as dispersal agents, especially if the virus were to change to a less lethal form through reassortment or mutation.
A key difference between mosquitoborne WNV and birdborne HPAI H5N1 is the virtual absence of effective reservoir hosts other than birds for the latter. WNV can be maintained without birds because infected mosquitoes can pass active virus to subsequent generations through vertical transmission (35). So far as is known, no alternative to birds exists as major reservoir hosts for HPAI H5N1.
An additional consideration concerning the future of HPAI H5N1, should it gain wide circulation in migratory birds, is the possibility of infection of a bird already infected with another form of avian influenza virus. Such infection could result in reassortment and production of a new virus, possibly less lethal than HPAI H5N1 but more readily spread.
HPAI H5N1 spread rapidly across Eurasia during 2005 for reasons that are not entirely understood. Despite this rapid movement, effective introduction (i.e., under conditions allowing its spread) of the virus to the New World through migratory or vagrant birds seems unlikely. Few individual members of few waterfowl species migrate between hemispheres, and should a bird make the journey while shedding sufficient active virus to infect birds in the Western Hemisphere, newly infected birds would probably die before being able to transport the virus from the entry site. If spread of HPAI H5N1 to the New World occurs in its current form (e.g., through domestic or pet bird trade or smuggling), it should be readily detectable because of the large number of dead native birds likely to result. However, the virus is changing (19), and a modified H5N1 virus introduced into the Western Hemisphere could be moved more readily by migratory waterfowl. If this event were to occur, the virus should be amenable to control through isolation and quarantine. If viral reassortment or mutation occurs to produce a new virus that is readily transmissible to humans, the role of birds in general and migrants in particular may be moot because of the fundamentally different methods of infection favored by viruses infecting humans and birds. Viruses infecting birds preferentially attack the intestinal tract and are shed with the feces; by contrast, human viruses mainly attack the respiratory tract and are shed with respiratory effluvia. If HPAI H5N1 were to gain wide circulation among migrants, it might infect a bird already infected with another form of avian influenza A and undergo reassortment to produce a low-pathogenic form that is more readily spread.
Dr Rappole is a research scientist with the Smithsonian National Zoological Park's Conservation and Research Center. His principal research interests are migratory bird ecology and evolution, sub-Himalayan ornithogeography, and avian conservation.
Dr Hubálek is a scientist at the Academy of Sciences of the Czech Republic. He is interested in the ecology of arthropodborne human pathogenic viruses and bacteria.
References
- Li KS, Guan Y, Wang J, Smith G, Xu K, Duan L, Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–13. DOIPubMedGoogle Scholar
- Zhou NN, Shortridge K, Claas E, Krauss S, Webster R. Rapid evolution of H5N1 influenza viruses in chickens in Hong Kong. J Virol. 1999;73:3366–74.PubMedGoogle Scholar
- Fauci AS. Pandemic influenza threat and preparedness. Emerg Infect Dis. 2006;12:73–7.PubMedGoogle Scholar
- Enserink M. H5N1 moves into Africa, European Union, deepening global crisis. Science. 2006;311:932. DOIPubMedGoogle Scholar
- Xu X, Subbarao K, Cox N, Guo Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology. 1999;261:15–9. DOIPubMedGoogle Scholar
- Morris R, Jackson R. Epidemiology of H5N1 avian influenza in Asia and implications for regional control. Rome: Food and Agricultural Organization of the United Nations; 2005.
- Sims LD, Ellis T, Liu K, Dyrting K, Wong H, Peiris M, Avian influenza in Hong Kong 1997–2002. Avian Dis. 2003;47:832–8. DOIPubMedGoogle Scholar
- Nguyen DC, Uyeki T, Jadhao S, Maines T, Shaw M, Matsuoka Y, Isolation and characterization of avian influenza viruses, including highly pathogenic H5N1, from poultry in live bird markets in Hanoi, Vietnam, in 2001. J Virol. 2005;79:4201–12. DOIPubMedGoogle Scholar
- Ng EK, Cheng P, Ng A, Hoang T, Lim W. Influenza A H5N1 detection. Emerg Infect Dis. 2005;11:1303–5.PubMedGoogle Scholar
- Ellis TM, Bousfield R, Bisset L, Dyrting K, Luk G, Tsim S, Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004;33:492–505. DOIPubMedGoogle Scholar
- Van Borm S, Thomas I, Hanquet G, Lambrecht B, Boschmans M, DuPont G, Highly pathogenic H5N1 influenza virus in smuggled Thai eagles, Belgium. Emerg Infect Dis. 2005;11:702–5.PubMedGoogle Scholar
- Chen H, Smith G, Zhang S, Qin K, Wang J, Li K, H5N1 virus outbreak in migratory waterfowl. Nature. 2005;436:191–2. DOIPubMedGoogle Scholar
- World Health Organization. H5N1 avian influenza timeline: 28 Oct 2005 [cited 2006 Oct 23]. Available from http://www.who.int/entity/csr/disease/avian_influenza/Timeline_28_10a.pdf
- Brown I, Gaidet N, Guberti V. Marangon, Olsen B, editors. Mission to Russia to assess the avian influenza situation in wildlife and the national measures being taken to minimize the risk of international spread. Office International des Epizooties 2005 [cited 2005 Oct 23]. Available from http://www.oie.int/downld/Missions/2005/ReportRussia2005Final2.pdf
- Office International des Epizooties. Update on avian influenza in animals (type H5) 24 May 2006 [cited 2006 May 24]. Available from http://www.oie.int/downld/AVIAN%20INFLUENZA/A_AI-Asia.htm
- Department for Environment, Food, and Rural Affairs (DEFRA), United Kingdom. Epidemiology report on avian influenza in a quarantine premises in Essex [cited 2006 May 29]. Available from http://www.defra.gov.uk/animalh/diseases/notifiable/disease/ai/pdf/ai-epidemrep111105.pdf
- World Health Organization (WHO). Cumulative number of confirmed human cases of avian influenza A/ (H5N1) reported to WHO as of 29 May 2006 [article in French] [cited 2006 May 29]. Available from http://www.who.int/csr/disease/avian_influenza/country/cases table_2006_05_29/en/index.html
- Webster RG, Bean W, Gorman O, Chambers T, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–79.PubMedGoogle Scholar
- Webster RG, Peiris M, Chen H, Guan Y. H5N1 outbreaks and enzootic influenza. Emerg Infect Dis. 2006;12:3–8.PubMedGoogle Scholar
- Kawaoka Y, Chambers T, Sladen W, Webster G. Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology. 1988;163:247–50. DOIPubMedGoogle Scholar
- Perkins LE, Swayne D. Comparative susceptibility of selected avian and mammalian species to a Hong Kong–origin H5N1 high-pathogenicity avian influenza virus. Avian Dis. 2003;47:956–67. DOIPubMedGoogle Scholar
- Shortridge KF, Zhou N, Guan Y, Gao P, Ito T, Kawaoka Y, Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–42. DOIPubMedGoogle Scholar
- Hulse-Post DJ, Sturm-Ramirez K, Humberd J, Seiler P, Govorkova E, Krauss S, Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc Natl Acad Sci U S A. 2005;102:10682–7. DOIPubMedGoogle Scholar
- Komar N, Langevin S, Hinten S, Nemeth E, Edwards D, Hettler B, Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–22.PubMedGoogle Scholar
- Munster VJ, Wallensten A, Baas C, Rimmelzwann G, Schutten M, Olsen B, Mallards and highly pathogenic avian influenza ancestral viruses, northern Europe. Emerg Infect Dis. 2005;11:1545–51.PubMedGoogle Scholar
- Spackman E, Stallknecht D, Slemons R, Winker K, Suarez D, Scott M, Phylogenetic analyses of type A influenza genes in natural reservoir species in North America reveals genetic variation. Virus Res. 2005;114:89–100. DOIPubMedGoogle Scholar
- Werner O. [Highly pathogenic avian influenza--a review.] [Article in German.]. Berl Munch Tierarztl Wochenschr. 2006;119:140–50.PubMedGoogle Scholar
- Kessel B, Gibson D. Status and distribution of Alaska birds. Studies in Avian Biology. 1978;1:1–100.
- Palmer R. Handbook of North American birds. Vol 2. New Haven (CT):Yale University Press; 1976.
- American Ornithologists' Union (AOU). The A.O.U. check-list of North American birds. 7th ed. Washington: American Ornithologists' Union; 1998.
- Rasmussen P, Anderton J. Birds of South Asia: the Ripley guide. Barcelona: Lynx Ed.; 2005.
- Rappole JH, Derrickson S, Hubálek Z. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg Infect Dis. 2000;6:319–28. DOIPubMedGoogle Scholar
- Bernard KA, Maffei J, Jones S, Kauffman E, Ebel G, Dupuis A II, West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg Infect Dis. 2001;7:679–85. DOIPubMedGoogle Scholar
- Brault AC, Langevin S, Bowen R, Panella N, Biggerstaff B, Miller B, Differential virulence of West Nile strains for American crows. Emerg Infect Dis. 2004;10:2161–8.PubMedGoogle Scholar
- Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742–7. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 12, Number 10—October 2006
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
John H. Rappole, 1500 Remount Rd, Front Royal, VA 22630, USA
Top