Volume 19, Number 1—January 2013
Research
Novel Polyomavirus associated with Brain Tumors in Free-Ranging Raccoons, Western United States
Abstract
Tumors of any type are exceedingly rare in raccoons. High-grade brain tumors, consistently located in the frontal lobes and olfactory tracts, were detected in 10 raccoons during March 2010–May 2012 in California and Oregon, suggesting an emerging, infectious origin. We have identified a candidate etiologic agent, dubbed raccoon polyomavirus, that was present in the tumor tissue of all affected animals but not in tissues from 20 unaffected animals. Southern blot hybridization and rolling circle amplification showed the episomal viral genome in the tumors. The multifunctional nuclear protein large T-antigen was detectable by immunohistochemical analyses in a subset of neoplastic cells. Raccoon polyomavirus may contribute to the development of malignant brain tumors of raccoons.
The American Cancer Society estimates that infectious pathogens are associated with up to 20% of all human cancers worldwide. Among oncogenic viruses are those in the Polyomaviridae family, whose members infect an array of vertebrate species, including birds, humans, nonhuman primates, bovids, rodents, and sea lions (1,2). Infection in mammals typically results in persistent asymptomatic infection (3,4). However, natural disease studies of human infection and experimental disease studies suggest that a potential outcome of some polyomavirus (PyV) infections is tumor formation (4–6). Experimental evidence that PyVs are tumorigenic is 50 years old and not debated; PyV-induced tumorigenesis in laboratory animals, by simian virus 40 (SV40) or by multiple human PyVs, such as JC virus (JCV), is used extensively as a cell transformation model. JCV, for example, induces brain tumors when intracerebrally inoculated in experimental animals (7–11). Furthermore, transgenic mice harboring the viral-encoded large T-antigen (LT-Ag) alone develop tumors of neuroectodermal origin, including malignant peripheral nerve sheath tumors (MPNSTs) and glioblastomas. Evidence that PyVs induce tumors after natural infection is accumulating but more controversial. Studies reliant on molecular detection of tumor-associated virus in isolation, however extensive, are inconclusive because association between PyVs and naturally occurring neoplasms varies and because PyV infections are highly prevalent, yet tumor formation is rare (3,4,12–14). Thus, although PyV-induced oncogenesis in laboratory animals has been a prolific model for the study of the cell cycle and cell transformation, natural infections rarely result in tumor formation, so the steps in cell transformation after natural infection are being revealed more slowly. Recent advances have been made by an accumulation of studies on Merkel cell polyomavirus (MCPyV), which is highly associated and integrated in most Merkel cell carcinomas (5). However, several unanswered questions relating to persistence, transmission, and transformation remain.
Most veterinary diagnostic laboratories receive large numbers of raccoon (Procyon lotor) carcasses for diagnosis, yet tumors of any type are rarely reported (15–17). In northern California and southern Oregon, we diagnosed 10 cases of olfactory tract/frontal lobe brain tumors in free-ranging raccoons during March 2010–May 2012. During the same period, no other raccoon tumors were reported in diagnostic laboratories across the United States and Canada. The clustering of cases and association of the tumors with the olfactory pathways suggested an infectious cause with a distinct route of transmission and tropism. We characterized from these tumors a candidate etiologic agent, raccoon polyomavirus (RacPyV).
Tissue Samples
All tissues were obtained by necropsy from the California Animal Health and Food Safety Laboratory, Veterinary Medical Teaching Hospital, at the School of Veterinary Medicine, University of California, Davis, California, or the Veterinary Diagnostic Library of Oregon State University, Corvallis, Oregon. DNA was extracted by using the DNeasy Blood and Tissue Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions.
Consensus PCR
Reactions were performed by using Phusion polymerase (New England Biolabs, Ipswich, MA, USA). Consensus PCR primers were designed on the basis of conserved stretches of amino acid sequences in multiple sequence alignments (Vector NTI, Invitrogen, Carlsbad, CA, USA) of LT-Ags from multiple PyV species. Primer sequences are listed in the Technical Appendix Table.
PCR conditions were as follows: 98°C for 10 sec, followed by 30 cycles of 98°C for 10 sec, 44°C for 40 sec, 72°C for 30 sec; then 72°C for 10 min; and a 4°C hold. Amplification products were subjected to electrophoresis on a 1% agarose gel, and amplicons were purified by using a QIAquick Gel Extraction Kit (QIAGEN).
Sequencing Reactions
PCR products were Sanger sequenced with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). This process was conducted on an ABI 3770 Genetic Analyzer (Applied Biosystems).
Phylogenetic Analysis
Reference viral sequences from the following PyVs were obtained from GenBank: JCV (accession no. J02226.1), SV40 (J02400.1), MCPyV (JF813003.1), murine polyomavirus (J02288.1), gorilla polyomavirus (HQ385752.1), chimpanzee polyomavirus (2c/6413) (HQ385751.1), chimpanzee polyomavirus (Azzie) (FR692336.1), and orangutan polyomavirus (FN356901.1). Amino acid sequence alignments of the predicted RacPyV LT-Ag, small T-antigen (sT-Ag), viral protein (VP) 1, and VP2 were generated by using ClustalW, implemented in MEGA5 (www.megasoftware.net). Aligned sequences were used to generate phylogenetic trees in MEGA5 by using the neighbor-joining method with amino acid p-distances and 1,000 bootstrap replicates. Intron splice sites in RacPyV LT-Ag were predicted by using the NetGene2 server (www.cbs.dtu.dk/services/NetGene2/).
Immunohistochemical Analysis
Tissue sections from the tumors and normal brain tissue from raccoons without tumors were sectioned at 4 μm thickness and placed on electromagnetically charged slides. Immunohistochemical analyses were performed by using the avidin-biotin peroxidase method as described (18). The primary antibodies used for this study included a mouse monoclonal antibody against a peptide from exon 2 of the MCPyV that specifically recognizes LT-Ag and 57-kDa isoforms but does not cross-react with sT-Ag (CM2B4; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a mouse monoclonal anti–wild-type p53 (Clone DO-7; Dako Laboratories, Carpinteria, CA, USA). Double labeling immunofluorescence was performed according to the protocol described above; however, secondary antibodies were tagged with Alexa-Fluor 488 for the LT-Ag primary and Alexa-Fluor 568 for the p53 primary (Invitrogen, Carlsbad, CA USA).
Southern Blot Hybridizations
Genomic DNA from tumor and normal brain tissue was extracted by using the DNeasy Blood and Tissue Kit (QIAGEN) according to the manufacturer’s instructions. RacPyV_LT_Probe1, a 799-bp digoxygenin-labeled probe designed to hybridize to the RacPyV LT-Ag coding region, was PCR amplified from RacPyV DNA according to the manufacturer’s instructions by using the PCR DIG Probe Synthesis Kit (Roche, Indianapolis, IN, USA) and verified (Technical Appendix Figure 1). One microgram DNA undigested or digested with KpnI at 37°C for 16 h was separated on a 0.8% agarose gel and transferred to positively charged nylon membranes (Roche) overnight by capillary action in 20× SSC. DNA was UV cross-linked and then hybridized with RacPyV_LT_Probe1 (Technical Appendix Figure 2). Hybridization occurred for 16 h at 48°C. Membranes were washed with washing buffer (0.1 mol/L maleic acid, 0.15 mol/L NaCl; pH 7.5; 0.3% [volume/volume] Tween 20) and developed with NBT/BCIP stock solution (Roche) for up to 2 h.
Rolling Circle Amplification
Rolling circle amplification (RCA) was conducted by using the illustra TempliPhi 100 Amplification Kit (GE Healthcare Life Sciences, Piscataway, NJ, USA) according to the manufacturer’s instructions. Briefly, 1 μL DNA extracted from tumor or normal brain tissue was added to 5 μL sample buffer and incubated for 5 min at 95°C. The sample was cooled on ice and then combined with 5 μL reaction buffer and 0.2 μL enzyme mix. The reaction was then incubated at 30°C for 16 h, followed by incubation at 56°C for 10 min to deactivate the polymerase. RCA amplified products were KpnI digested at 37°C for 16 h and separated on a 0.8% agarose gel.
Clinical Findings and Tumor Pathology
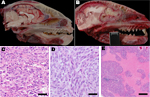
During March 2010–May 2012, 52 raccoons were submitted to the California Animal Health and Food Safety Laboratory at the University of California, Davis, for full necropsy, with rabies screening performed as part of state diagnostic protocol. Ten (19%) raccoons had brain tumors within the cranial portion of their frontal lobe(s), most of which spanned the cribiform plate of the ethmoid bone and extended into, or from, the olfactory tract (Figure 1). Nine of the affected animals represented separate collection events from 3 adjacent counties in California, and 1 affected animal was shipped from southern Oregon. In all instances, the raccoons were exhibiting neurologic signs, including wandering in the daylight, approaching humans, or exhibiting lack of consciousness. Overall, other tissues, including lymphoid and hematopoietic tissues such as bone marrow, thymus, lymph nodes, and spleen, were generally within normal limits.
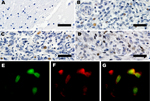
In one of the affected raccoons (Rac7), a presumed early (grossly undetectable) tumor was exclusively localized within the olfactory tract and segments of the axonal bundles of olfactory nerves within the ethmoid turbinates, which makes this the likely site of tumor origin. Most of the tumors were histologically pleomorphic, and each of the 10 tumors have been alternately and preliminarily classified as MPNSTs or glioblastomas on the basis of criteria such as anatomic location, histopathology (Figure 1), and a panel of histochemical and immunohistochemical stains, including reticulin stain, glial fibrillary acidic protein, vimentin, pancytokeratin, neuron-specific nuclear protein, Olig2, synaptophysin, and laminin. Among consistent findings in the glial tumors was expression of glial fibrillary acidic protein, variably sized regions of necrosis (Figure 1), and high mitotic activity. Tumors classified as MPNSTs also were positive for glial fibrillary acidic protein; however, neoplastic cells were individually surrounded by a reticulin-positive framework, which was immunoreactive with laminin. Viral inclusions were not detectable histologically, and no viral particles were visualized by transmission electron microscopy.
RacPyV Discovery by Consensus PCR
JCV is a human PyV that has been associated with brain tumors, including a broad range of glial-origin tumors. In experimental studies, transgenic mice expressing JCV LT-Ag under the control of the Mad-4 promoter develop MPNSTs and under the regulation of the CY (archetype) promoter develop medulloblastomas and glioblastomas (19). Because LT-Ag is a principal PyV protein that orchestrates oncogenesis (13,20–25), we chose it as the target for consensus PCR detection. Using an alignment of 21 PyVs, we designed 4 sets of degenerate primers to amplify conserved regions within LT-Ag (Technical Appendix Figure 3). Amplification was successful by using primers PLMA_F3 and PLMA_R1, yielding a 270-bp product from tumor tissue of the 3 raccoons originally tested. Thus far, the virus has not been detected in brain tissue or multiple other tissues (kidney, spleen, feces, salivary gland, lung, tonsil, nasal swab) tested in 20 unaffected animals collected from the same geographic region. Full length viral genomes (6/10) or partial genomes (4/10, ranging from 2,998 bp to 4,667 bp) were generated by using a combination of primer walking and specific RacPyV primers.
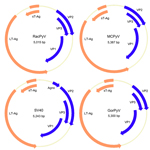
RacPyV Genome Analysis
The novel virus was most closely related, phylogenetically, to a clade of viruses that includes MCPyV and was named raccoon polyomavirus (RacPyV). PyVs are small, circular, double-stranded DNA viruses, and analysis of the RacPyV genome showed characteristic organization for Polyomaviridae (Figure 2) (6). RacPyV encodes putative open reading frames (ORFs) with homology to PyV LT-Ag, sT-Ag, the structural VPs 1–3, and a single bidirectional noncoding regulatory region (NCRR). Because of sequence variation among the raccoon isolates, the designations 1–10 indicate the order of animal submission and which raccoon harbored which respective RacPyV; that is, RacPyV1 was found in Rac1 and was the first tumor and RacPyV discovered.
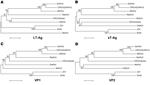
Phylogenetic trees of the deduced PyV proteins constructed by using the neighbor-joining method show that the putative early and late proteins of RacPyV are most closely related to those of MCPyV and chimpanzee PyV (Figure 3). The 10 RacPyV genomes had a total of 30 single nucleotide polymorphisms (SNPs) (Figure 4). Most (29/30) of the SNPs were synonymous substitutions or in noncoding regions, as seen with strain variants of other PyVs. Half (15/30) of the SNPs were unique to the single Oregon case (Rac6), perhaps reflecting its geographic distance from the California raccoons. Three distinct nucleotide deletion events occurred across RacPyV5 and RacPyV10. RacPyV5 had a 26-bp deletion within the putative LT-Ag intron and a 12-bp deletion within the putative NCRR; RacPyV10 had a 1-bp deletion within the putative LT-Ag intron. The genome of RacPyV3 is deposited in GenBank (accession no. JQ178241).
The NCRR of RacPyV encodes multiple consensus pentanucleotide LT-Ag binding sites (GAGGC) on both strands as seen in other PyVs. RacPyV LT-Ag has 46% homology to MCPyV LT-Ag and has features conserved among PyV LT-Ags, including the LXCXE motif necessary for LT-Ag association with the retinoblastoma protein and the Walker A box GPXXXGKT, which suggests that RacPyV LT-Ag is an ATPase that binds to p53 (26). In experimental models, these sites are crucial components of virus-mediated transformation (20,22,23).
The putative ORF encoding LT-Ag in RacPyV encodes putative splice sites surrounding a 387-bp intron. In other PyVs, sT-Ag, which shares a start site with LT-Ag, promotes cell transformation through negative regulation of the protein phosphatase 2A (PP2A) family of serine-threonine phosphatases (26,27). In transcription and expression studies, sT-Ag terminates at a stop codon within the LT-Ag intron. Aside from chimpanzee PyV (Azzie isolate) (28), all PyVs discovered thus far contain this canonical intronic stop codon that defines the ORF for sT-Ag. However, among the 7 RacPyVs for which the intron of LT-Ag was completely sequenced, only 2 (RacPyV5 and RacPyV10) contain intronic in-frame stop codons that would define an independent sT-Ag. In the 5 other RacPyVs, the entire T-antigen coding region is in-frame as a putative single ORF. Although expression studies are needed, among possible explanations are that a single T-Ag contains both LT-Ag and sT-Ag domains, that sT-Ag could be expressed as an alternately spliced form of the T-Ag coding region, that sT-Ag is generated proteolytically, or that it is not expressed. Of the 2 viruses that have a stop codon in-frame within the LT-Ag intron, RacPyV10 has a stop codon that terminates sT-Ag after the PP2A domains, as is seen in other PyVs, whereas RacPyV5 encodes a stop codon before the PP2A domains. However, the sT-Ag of MCPyV constructs altered to lack a functional PP2A binding domain still initiates cell transformation (29).
Episomal RacPyV
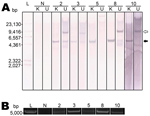
Southern blot hybridizations and RCA were performed to ascertain whether RacPyV existed in episomal, integrated, or a combination of these forms. Viral DNA of sufficient quantity and quality was present in 5/10 tumors for these assays. In all cases, an ≈5-kb band for the KpnI-digested samples was absent or present faintly in undigested conditions (Figure 5, panel A, closed arrow). The band variably present at ≈9 kb is expected to be a circular nicked form of the genome (Figure 5, panel A, open arrow). RCA, by using random hexamers, was performed on DNA extracted from the same set of tumor tissues. RCA products of the tumor tissues produced the expected ≈5-kb band after KpnI digestion, confirming the Southern blot result that RacPyV is not detectably integrated in the host genome and demonstrating that it exists in circular form in tumor cells (Figure 5, panel B). As expected, Southern blot and RCA analyses performed on DNA extracted from normal, unaffected raccoon brain tissue produced no bands (Figure 5, panels A, B).
Expression of RacPyV LT-Ag in the Nuclei of Tumor Cells
To determine the expression of LT-Ag, we performed immunohistochemical analyses with a monoclonal antibody that recognizes a peptide within exon 2 of MCPyV LT-Ag. The LT-Ag was absent (Figure 6) in unaffected regions of the brain and absent in unaffected raccoon tissues but was detected within the nuclei of a subset of neoplastic cells in 4 tested raccoon tumors (Figure 6). Immunohistochemical analyses for wild-type p53 also was positive in a subset of neoplastic cells (Figure 6) but was negative in the adjacent normal brain. Furthermore, double-labeling immunofluorescence showed the co-localization of LT-Ag with wild-type p53 in the nuclei of neoplastic cells (Figure 6).
We consider this an outbreak of brain tumors because a retrospective case search and literature review of ≈700 necropsies from California and other regions of the United States and Canada showed only 2 primary brain tumors in raccoons, both classified as low-grade astrocytomas (30,31). Because of the consistent anatomic location of the tumors, the absolute association with RacPyV, and the expression of LT-Ag, we propose that RacPyV might play a role in tumorigenesis. In our sampling of tissues from affected and unaffected raccoons, viral genomic sequences were limited to the neoplastic tissue of the brain and olfactory tract. A more extensive set of samples might uncover RacPyV viremia and shedding sites, but it is also worth considering that the raccoon might be a dead-end host for viral replication and that viral persistence and shedding might occur in a different species. Raccoons in the affected geographic region have a nomadic suburban lifestyle and use sewer and water lines for travel and presumed daily exposure to urine, garbage, environmental toxins, and environmental pathogens, including PyVs, which have been consistently detected in raw urban sewage samples worldwide (32).
Compared with other PyVs, RacPyV has several unique features. sT-Ag, for example, which in SV40 and other PyVs contributes to cell transformation (27,29,33), had a predicted termination site within the LT-Ag intron in only 2/7 RacPyVs analyzed. RacPyV has no predicted ORF or sequence homology for the Agno gene, which is found in many PyVs. RacPyV does not appear to be integrated into the host genome because both Southern blot hybridization and RCA analyses indicate that full-length genome exists in an episomal, circular state. Integration is one method of achieving replication incompetence of PyV and is considered by many researchers to precede cancer development (34). However, PyVs have alternate mechanisms of replication incompetence, and late gene suppression might occur in episomal virus. For example, Carbone et al. have implicated antisense RNA molecules produced in SV40 infection in late gene silencing through Dicer-mediated degradation (35). Natural and experimental infections with PyVs result in a spectrum of tumors that have some distinctions on the basis of route of infection, cofactors, and/or viral tropism (7,8,10,11). If RacPyV is driving oncogenesis, the transformation location has been remarkably consistent.
In humans, onset of viral-associated cancer typically occurs decades after initial infection with a transforming virus; however, free-ranging raccoons are short lived (≈2–3 years), so this naturally occurring transformation event must be relatively rapid. Natural models of viral-induced tumorigenesis provide an excellent opportunity to analyze mechanisms of transmission, transformation, viral persistence, and routes of shedding and infection. Raccoons are undeniably successful in the human suburban environment and have been proposed as a sentinel species for environmental contaminants and exposure to pathogens (36). New animal PyV species have been identified frequently during the last few years, but the strong association of RacPyV with a relatively sudden occurrence of brain tumors distinguishes it from other PyVs, and its discovery could help shed light on the etiologic role of PyVs in oncogenesis.
Mr Dela Cruz, Jr., is a research assistant in in the Department of Pathology, Microbiology, and Immunology, School of Veterinary Medicine, at the University of California, Davis. His research interests include use of biomolecular methods to understand viral pathogenesis and discovery.
Acknowledgments
We appreciate the help of Hongwei Liu and the California Animal Health and Food Safety histology laboratory. Deanna Clifford, California Fish and Game, Wildcare of Marin, Lindsay Wildlife Museum, and Sonoma Wildlife Rescue supported and submitted the northern California cases, and Howard Gelberg kindly supplied tissue from the case he identified in Oregon. We are also indebted for manuscript edits and conversations with David Clark and Peter Barry.
Viral discovery in the Pesavento laboratory is generously funded by the Bernice Barbour Foundation. L.L. and E.D. were supported by the Blood Systems Research Institute and the National Heart, Lung, and Blood Institute, gran no. R01 HL105770.
References
- Johne R, Buck CB, Allander T, Atwood WJ, Garcea RL, Imperiale MJ, Taxonomical developments in the family Polyomaviridae. Arch Virol. 2011;156:1627–34. DOIPubMedGoogle Scholar
- Colegrove KM, Wellehan JF Jr, Rivera R, Moore PF, Gulland FM, Lowenstine LJ, Polyomavirus infection in a free-ranging California sea lion (Zalophus californianus) with intestinal T-cell lymphoma. J Vet Diagn Invest. 2010;22:628–32. DOIPubMedGoogle Scholar
- Rollison DE. Epidemiologic studies of polyomaviruses and cancer: previous findings, methodologic challenges and future directions. Adv Exp Med Biol. 2006;577:342–56. DOIPubMedGoogle Scholar
- Poulin DL, DeCaprio JA. Is there a role for SV40 in human cancer? J Clin Oncol. 2006;24:4356–65. DOIPubMedGoogle Scholar
- Chang Y, Moore PS. Merkel cell carcinoma: a virus-induced human cancer. Annu Rev Pathol. 2012;7:123–44. DOIPubMedGoogle Scholar
- Imperiale M, Major E. Polyomaviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields virology, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 2263–98.
- Walker DL, Padgett BL, ZuRhein GM, Albert AE, Marsh RF. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973;181:674–6. DOIPubMedGoogle Scholar
- London WT, Houff SA, Madden DL, Fuccillo DA, Gravell M, Wallen WC, Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus). Science. 1978;201:1246–9. DOIPubMedGoogle Scholar
- Ohsumi S, Ikehara I, Motoi M, Ogawa K, Nagashima K, Yasui K. Induction of undifferentiated brain tumors in rats by a human polyomavirus (JC virus). Jpn J Cancer Res. 1985;76:429–31 .PubMedGoogle Scholar
- Del Valle L, Gordon J, Ferrante P, Khalili K. JC virus in experimental and clinical brain tumors. In: Khalili K, Stoner G, editors. Human polyoviruses molecular and clinical perspectives. New York: Wiley-Liss, Inc.; 2001. p. 409–30.
- Barbanti-Brodano G, Martini F, De Mattei M, Lazzarin L, Corallini A, Tognon M. BK and JC human polyomaviruses and simian virus 40: natural history of infection in humans, experimental oncogenicity, and association with human tumors. Adv Virus Res. 1998;50:69–99. DOIPubMedGoogle Scholar
- White MK, Gordon J, Reiss K, Del Valle L, Croul S, Giordano A, Human polyomaviruses and brain tumors. Brain Res Brain Res Rev. 2005;50:69–85. DOIPubMedGoogle Scholar
- Pipas JM. SV40: cell transformation and tumorigenesis. Virology. 2009;384:294–303. DOIPubMedGoogle Scholar
- Lednicky JA, Butel JS. Polyomaviruses and human tumors: a brief review of current concepts and interpretations. Front Biosci. 1999;4:d153–64. DOIPubMedGoogle Scholar
- Hamir AN. Pathology of neurologic disorders of raccoons (Procyon lotor). J Vet Diagn Invest. 2011;23:873–84. DOIPubMedGoogle Scholar
- Mikaelian I, Martineau D, Helie P, Patenaude R, Campbell D, Barker IK. Tumors in wild adult raccoons from a suburban area. Can Vet J. 1999;40:429–30 .PubMedGoogle Scholar
- McAloose D, Newton AL. Wildlife cancer: a conservation perspective. Nat Rev Cancer. 2009;9:517–26. DOIPubMedGoogle Scholar
- Del Valle L, Gordon J, Assimakopoulou M, Enam S, Geddes JF, Varakis JN, Detection of JC virus DNA sequences and expression of the viral regulatory protein T-antigen in tumors of the central nervous system. Cancer Res. 2001;61:4287–93 .PubMedGoogle Scholar
- Krynska B, Otte J, Franks R, Khalili K, Croul S. Human ubiquitous JCV(CY) T-antigen gene induces brain tumors in experimental animals. Oncogene. 1999;18:39–46. DOIPubMedGoogle Scholar
- Caracciolo V, Reiss K, Khalili K, De Falco G, Giordano A. Role of the interaction between large T antigen and Rb family members in the oncogenicity of JC virus. Oncogene. 2006;25:5294–301. DOIPubMedGoogle Scholar
- Frisque RJ, Hofstetter C, Tyagarajan SK. Transforming activities of JC virus early proteins. Adv Exp Med Biol. 2006;577:288–309. DOIPubMedGoogle Scholar
- Reich NC, Levine AJ. Specific interaction of the SV40 T antigen-cellular p53 protein complex with SV40 DNA. Virology. 1982;117:286–90. DOIPubMedGoogle Scholar
- Dyson N, Bernards R, Friend SH, Gooding LR, Hassell JA, Major EO, Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol. 1990;64:1353–6 .PubMedGoogle Scholar
- Del Valle L, Gordon J, Assimakopoulou M, Enam S, Geddes JF, Varakis JN, Detection of JC virus DNA sequences and expression of the viral regulatory protein T-antigen in tumors of the central nervous system. Cancer Res. 2001;61:4287–93 .PubMedGoogle Scholar
- Del Valle L, Pina-Oviedo S, Perez-Liz G, Augelli BJ, Azizi SA, Khalili K, Bone marrow-derived mesenchymal stem cells undergo JCV T-antigen mediated transformation and generate tumors with neuroectodermal characteristics. [Epub ahead of print]. . Cancer Biol Ther. 2010;9. DOIPubMedGoogle Scholar
- Pipas JM. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992;66:3979–85 .PubMedGoogle Scholar
- Sablina AA, Hahn WC. SV40 small T antigen and PP2A phosphatase in cell transformation. Cancer Metastasis Rev. 2008;27:137–46. DOIPubMedGoogle Scholar
- Deuzing I, Fagrouch Z, Groenewoud MJ, Niphuis H, Kondova I, Bogers W, Detection and characterization of two chimpanzee polyomavirus genotypes from different subspecies. Virol J. 2010;7:347. DOIPubMedGoogle Scholar
- Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E–BP1 translation regulator. J Clin Invest. 2011;121:3623–34. DOIPubMedGoogle Scholar
- Diters RW, Kircher CH, Nielsen SW. Astrocytoma in a raccoon. J Am Vet Med Assoc. 1978;173:1152–3 .PubMedGoogle Scholar
- Hamir AN, Picton R, Blythe LL, Heidel JR. Diagnostic exercise: astrocytoma with involvement of medulla oblongata, spinal cord, and spinal nerves in a raccoon (Procyon lotor). Vet Pathol. 2008;45:949–51. DOIPubMedGoogle Scholar
- Bofill-Mas S, Pina S, Girones R. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl Environ Microbiol. 2000;66:238–45. DOIPubMedGoogle Scholar
- Yu J, Boyapati A, Rundell K. Critical role for SV40 small-t antigen in human cell transformation. Virology. 2001;290:192–8. DOIPubMedGoogle Scholar
- zur Hausen H. The search for infectious causes of human cancers: where and why. Virology. 2009;392:1–10. DOIPubMedGoogle Scholar
- Carbone M, Pannuti A, Zhang L, Testa JR, Bocchetta M. A novel mechanism of late gene silencing drives SV40 transformation of human mesothelial cells. Cancer Res. 2008;68:9488–96. DOIPubMedGoogle Scholar
- Bigler WJ, Jenkins JH, Cumbie PM, Hoff GL, Prather EC. Wildlife and environmental health: raccoons as indicators of zoonoses and pollutants in southeastern United States. J Am Vet Med Assoc. 1975;167:592–7 .PubMedGoogle Scholar
Figures
Cite This ArticleTable of Contents – Volume 19, Number 1—January 2013
| EID Search Options |
|---|
|
|
|
|
|
|
![Thumbnail of Partial genome sequences (ranging from 2,998 bp in raccoon polyomavirus 6 [RacPyV6] to 4,667 bp in RacPyV9) were obtained from 4 raccoons that had undergone prolonged storage (RacPyV9) or were available only as formalin-fixed, paraffin-embedded tissue (dashed line, RacPyVs 1, 6, and 7). Gaps in the sequences correspond to regions where amplification reactions failed. The genomes of RacPyVs 2, 3, 4, 5, 8, and 10 were sequenced in their entirety by using a primer walking method to com](/eid/images/12-1078-F4-tn.jpg)
Please use the form below to submit correspondence to the authors or contact them at the following address:
Patricia A. Pesavento, Department of Pathology, Microbiology & Immunology, School of Veterinary Medicine, University of California, Davis, Davis, CA 95616, USA
Top