Volume 12, Number 9—September 2006
Research
Periurban Trypanosoma cruzi–infected Triatoma infestans, Arequipa, Peru
Abstract
In Arequipa, Peru, vectorborne transmission of Chagas disease by Triatoma infestans has become an urban problem. We conducted an entomologic survey in a periurban community of Arequipa to identify risk factors for triatomine infestation and determinants of vector population densities. Of 374 households surveyed, triatomines were collected from 194 (52%), and Trypanosoma cruzi–carrying triatomines were collected from 72 (19.3%). Guinea pig pens were more likely than other animal enclosures to be infested and harbored 2.38× as many triatomines. Stacked brick and adobe enclosures were more likely to have triatomines, while wire mesh enclosures were protected against infestation. In human dwellings, only fully stuccoed rooms were protected against infestation. Spatially, households with triatomines were scattered, while households with T. cruzi–infected triatomines were clustered. Keeping small animals in wire mesh cages could facilitate control of T. infestans in this densely populated urban environment.
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, causes more deaths in the Americas than any other parasitic disease (1). T. cruzi is carried in the gut of bloodsucking triatomine insects (Hemiptera, Reduviidae), and the parasite is usually transmitted to humans when the vector's feces enter the host through the insect bite or mucous membranes (2). Triatoma infestans is the principal vector of T. cruzi in the southern cone of South America and the sole vector in southern Peru. It is a highly synanthropic insect found most often in poor, rural households (3,4). However, in Arequipa, a city of 850,000 inhabitants in the arid highlands of southern Peru, T. infestans and T. cruzi have become periurban and urban problems.
Since 1991, T. infestans has been the target of an elimination program known as the Southern Cone Initiative (5). Member countries of this initiative have controlled or eliminated transmission of Chagas disease by spraying households with pyrethroid insecticides (6–9). In 2002, the Arequipa Regional Ministry of Health began a spray-based vector control program after an infant in a periurban community died from acute Chagas disease. This initiative, in contrast to those in other parts of the southern cone, is concentrated in and around the city rather than in rural areas. Novel measures may be necessary to prevent vector reinfestation after insecticide application in densely populated environments.
Urbanization of T. infestans has been observed elsewhere in South America (10–12), and other Chagas disease vectors have been observed in cities (13,14). Nevertheless, little is known about the epidemiology of Chagas disease transmission in and around cities. To tailor vector control strategies for the urban setting, we conducted a study to identify determinants of triatomine infestation and population density in a periurban community of Arequipa. We also examined triatomines for T. cruzi to gain a better understanding of the spatial distribution of potential Chagas disease transmission in the community.
Study Area and Population
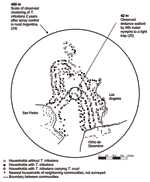
Arequipa is located at an elevation of 2,300 m at the foot of an active volcano (16.44° S, 71.59° W). The area is arid; rain is scarce and falls almost exclusively from December through February. Santa Maria de Guadalupe and Alto Guadalupe (hereafter referred to together as Guadalupe) are 2 of hundreds of communities located on hillsides on the outskirts of Arequipa (Figure 1). The communities are pueblos jovenes, settlements built by displaced families, many of whom migrated from rural areas to the city out of economic necessity after agrarian reform in 1969. Migrants relocated to pueblos jovenes in Arequipa in even greater numbers to escape terrorism from 1980 to 1995 (15). In preliminary analyses of survey data from 1,444 schoolchildren living in Guadalupe and surrounding communities, 71 (4.9%) had serologic evidence of T. cruzi infection (N. Bowman, pers. comm.). The community of Guadalupe consists of 397 dwellings that house ≈2,550 people in an area of 14.1 ha (2,800 households/km2). Typical households consist of a human dwelling (bedrooms, kitchens, living rooms, and storage rooms) plus an enclosed yard. Roofs of the human dwellings are fully stuccoed or of corrugated metal. Walls consist of a wide variety of materials including sillar, a white, porous rock of compounded volcanic ash. Most yards share stone walls with neighboring households, though some back directly up against the basalt (a volcanic stone) rubble of the steep hillside. Neither community underwent systematic insecticide application before this study.
Study Design
The entomologic survey was conducted in coordination with the first round of household insecticide application by the Arequipa Ministry of Health Vector Control Program, from November 15 to December 8, 2004. Ministry of Health personnel sprayed each house and all peridomestic structures with deltamethrin powder suspended in water at a rate of 25 mg/m2 (K-othrine, Bayer, Lima, Peru). After insecticide application, 2 trained triatomine collectors systematically searched each room of the human dwelling, animal enclosure, and remaining peridomestic area for a total of 1 person-hour. Because pilot studies showed marked variation in vector infestation and density within dwellings, data were collected at the level of individual rooms and animal enclosures. An adult from each household responded to a structured questionnaire regarding insecticide usage, cleaning practices, and potential triatomine hosts in each room of the dwelling and each animal enclosure. A collector recorded all construction materials used for each site. Household position was determined with a handheld global positioning system unit with an accuracy of 10 m (Garmin Corporation, Olathe, KS, USA). The protocol was reviewed by the Centers for Disease Control and Prevention's institutional review board.
Triatomines captured from each site were stored separately on ice packs until processing at the National University of San Agustin. Vectors were counted by site, stage, and sex (for adults). Live and moribund fifth instar and adult triatomines were examined for T. cruzi consecutively for each site until 1 positive insect was found, 10 negative insects had been examined, or all available insects had been examined, whichever came first. The sampling scheme was designed to detect T. cruzi in each site of collection with 80% power if >20% of insects were infected. We followed the procedures for examining triatomines for T. cruzi outlined in Gürtler et al. (16). Briefly, intestinal contents of the insects were extracted by applying pressure to the lower abdomen of the triatomine with forceps. Extracted material was then diluted in 1 drop of saline solution and examined under a microscope at 400× magnification.
Data Analysis
Two outcome variables were examined: T. infestans presence (a binary outcome) and T. infestans population density as estimated by the number of insects captured in 1 person-hour (a continuous count outcome). Each outcome was examined separately for rooms in human dwellings and animal enclosures. In univariate analyses, associations between triatomine presence and independent variables were evaluated with the χ2 test for binary variables and Kruskal-Wallis trend test for ordinal and continuous variables. All variables with p value <0.20 in univariate analyses, as well as other likely confounders, were considered in multivariate analyses (17). Multivariate models were fit with generalized estimating equations (GEEs). A spatial variogram was used to guide selection of a correlation structure for the GEE analysis (18). In the absence of correlation among observations from adjacent households, an exchangeable correlation structure was assumed to adjust confidence intervals for repeated observations from the same household. Nonsignificant variables were dropped sequentially from the multivariate models on the basis of their Wald scores. Analyses were performed in Stata version 8 (StataCorp LP, College Station, TX, USA) and R version 2.1 (http://www.r-project.org).
Analyses of estimated triatomine density were limited to infested rooms and animal enclosures. To compare the mean number of vectors captured during each timed search, we used zero-truncated negative binomial regression, a method appropriate for analyses of count data in which observations of zero are excluded (19). Because the data were overdispersed, the zero-truncated negative binomial distribution fit the data better than the zero-truncated Poisson distribution based on the likelihood-ratio test. GEE methods for zero-truncated negative binomial regression are not available. Therefore, for households with >1 infested room or animal enclosure, 1 site only, selected at random, was included in each analysis to maintain independence of observations. All variables with p<0.20 in univariate analyses and other likely confounders were considered for inclusion in a multivariate models of the same type. Analyses were performed in Stata version 8.0.
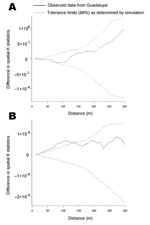
The spatial K function of Ripley was used to test for spatial clustering of infested households (20). Conceptually, the K function measures the expected number of households within a set distance of any given household. The difference between the K function that summarized spatial distribution of T. infestans–positive households and the K function that summarized the distribution of T. infestans–negative households was calculated. A difference in K functions of greater than zero suggests spatial clustering of positive households (21). The analysis was repeated at 30 spatial scales from 10 to 300 m, and for each spatial scale, 99% tolerance limits around the observed difference in K functions for positive and negative households were determined through simulation (21,22). Clustering of households with T. cruzi–positive T. infestans was assessed in the same manner. A weighted version of the spatial K function was used to test for clustering of the number of triatomines caught in each house (23).
Of 397 households in Guadalupe, 374 (94.2%) were sprayed and surveyed, and 194 (52.0%) were infested with triatomines (Figure 2). Seventy-two households (19.3%) harbored triatomines infected with T. cruzi. Triatomines and T. cruzi–infected triatomines were present in human dwellings and peridomestic areas. Of 1,424 rooms in human dwellings surveyed, 241 (17%) were infested with vectors and 54 (3.8%) contained T. cruzi–infected triatomines. Of 803 animal enclosures, 107 (13%) were infested with vectors and 31(3.9%) had insects infected with parasites. A total of 5,398 triatomines were captured, 2,270 in human dwellings and 3,128 in peridomestic areas. The colonization indices (number of sites with triatomine nymphs/number of sites with triatomines) for rooms and animal enclosures were 76% and 93%, respectively.
At the time of the survey, 263 (70.0%) households in Guadalupe kept domestic animals. We recorded 1,700 guinea pigs, 1,295 rabbits, 819 chickens, 469 sheep, 358 dogs, 135 cats, 126 turkeys, 70 cows, and 34 pigs. Stuccoed enclosures generally housed guinea pigs (29 of 34, correlation coefficient 0.30), chicken-wire enclosures housed rabbits (54 of 134, correlation coefficient 0.18), and adobe enclosures housed sheep (15 of 39, correlation coefficient 0.17). Other types of enclosures contained a range of animals, but none predominated (all correlation coefficients <0.15). Most animals, including large animals, were kept in enclosures on the roof or in the yard of the household; only 66 households (18.7%) allowed some companion animals to sleep inside at night.
T. infestans in Animal Enclosures
Several potential risk factors for triatomine infestation in animal enclosures were identified in univariate analyses (Table 1). In the multivariable logistic model, wire mesh enclosures were one fifth as likely to be positive than were enclosures of all other materials. Enclosures built wholly or partially of stacked brick or adobe were significantly more likely to be infested than other enclosures. Mortar between units of stone or brick did not significantly reduce the likelihood of infestation. Guinea pig enclosures were 1.69 times as likely to harbor vectors than were other enclosures; chicken enclosures were significantly less likely to be infested than were other enclosures. All 34 fully stuccoed enclosures were negative for T. infestans and were omitted from multivariate analyses.
Analyses of triatomine population density included 76 infested enclosures, each from a different household. The average number of insects caught per enclosure was 21.9. Enclosures with guinea pigs had an average of 33.9 insects; wire mesh enclosures averaged 6.7 insects (Table 2). In the multivariate zero-truncated negative binomial model, the presence of guinea pigs was associated with a 2.4-fold increase in estimated triatomine density, and wire mesh enclosures were estimated to harbor only 9% as many triatomines as enclosures of any other material. Some materials in which insect collection was difficult showed lower vector densities.
T. infestans in Rooms of the Human Dwelling
In the multivariate logistic model, the relative odds of infestation in rooms of the human dwelling increased multiplicatively by 1.6 for each additional person sleeping in a room (Table 3). Rooms in which an animal slept were nearly twice as likely as rooms without animals to be positive. Likelihood of being infested was less than one third for fully stuccoed rooms, 1.6 times greater for rooms built with mortared sillar, and 1.8 times greater for those built with mortared brick. An infested guinea pig enclosure and yard were both associated with infestation inside the human dwelling.
A random selection of 156 infested rooms, each from a different household, was included in the analysis of vector density (Table 4). Rooms had significantly lower estimated vector densities than did animal enclosures (Kruskal-Wallis p = 0.0001); the overall average number of insects collected in rooms was 8.9. In the multivariate model, the number of insects captured increased by 42% for each person sleeping in the room. Rooms in which animals, mainly dogs and cats, slept had an estimated 5.2 times as many insects as rooms without animals. Rooms with fully stuccoed or adobe walls had significantly fewer triatomines than rooms made of other materials. The density of vectors in rooms of mortared brick, basalt, or sillar was not significantly different from that in other rooms after controlling for covariates.
Spatial Analysis
Triatomine-infested households were not significantly clustered at any of the spatial scales examined (Figure 3A). We saw no evidence of clustering in the estimated population density of triatomines across the study site. Households with triatomines infected with T. cruzi, however, were significantly more clustered than households without infected insects. The difference in K functions exceeded 99% tolerance limits at all but 1 spatial scale (20 m) from 10 to 140 m (Figure 3B).
Elimination of T. infestans from Arequipa may be impeded by the ease with which the vector can return to sprayed households in the densely populated urban environment. Just 2 years after blanket deltamethrin spraying in a rural community in Argentina, T. infestans was found in sites clustered within 450 m of a putative source population (24). If the range of T. infestans redispersion is similar in Guadalupe, a single residual population of vectors would put every household in the community, as well as many households in 3 neighboring communities, at risk for vector reinfestation. T. infestans nymphs can walk >42 m (25) and can easily climb across or through crevices in the stone walls separating the densely packed houses of Guadalupe (2,800 households/km2). In the rural Argentina site, with 42 households/km2 (26), vector redispersion was thought to be through flight of adult insects (24). T. infestans adults usually fly only under specific conditions (25). In densely populated urban and periurban sites, walking is likely to be the principal mode of redispersion, and reinfestation is likely to be much more rapid than in rural settings.
Vector reinfestation typically begins in the peridomestic environment, where domestic animals are kept (27). The guinea pig, a staple source of protein in the Andes for thousands of years (28), and reportedly a reservoir of Chagas disease in Peru (29–31), is the most numerous domestic animal in Guadalupe. As our data demonstrate, guinea pig presence is also a determinant of peridomestic T. infestans infestation. Enclosures where guinea pigs were housed were more likely to be infested and, when infested, harbored twice as many vectors as other enclosures. Schofield hypothesizes that T. infestans population growth slows when host protective behavior, such as scratching and swatting, limit the insects' ability to complete a blood meal (32,33). Incompletely fed triatomines have delayed molting (34) and are more likely to migrate (25,35); vector population sizes thereby decrease without an increase in insect deaths (33). Compared with other animals, guinea pigs may be less able to repel feeding vectors. Their habit of pressing against enclosure walls may also facilitate triatomine feeding and increase vector population growth.
Chickens are associated with infestation and increased triatomine density in rural settings, where they typically range and roost freely (4,36). Cecere et al. suggest that confining chickens might reduce triatomine populations (36); in Guadalupe, space constraints force most households to keep chickens enclosed. The result seems to be a decrease in the importance of chickens as hosts for T. infestans. Chickens hunt triatomines by sight (34) and may be more able to detect and catch insects from the walls of urban enclosures than from roosting materials in rural settings.
The materials used to build animal enclosures were stronger predictors of T. infestans presence than were the type of animals housed in the enclosure. Many materials cheaply or freely available in Arequipa, such as unmortared brick, sillar, and basalt, provide ample refuge for insects. Adobe was less common but was also associated with an increased risk for infestation. Fully stuccoed enclosures were never infested in Guadalupe but are costly to build. Replacing small animal enclosures of brick, sillar, basalt, and adobe with inexpensive wire mesh structures, which are refractory to triatomine colonization, may be the most feasible intervention to slow or prevent vector reinfestation.
The presence and density of triatomines in rooms of human dwellings are critical determinants of the risk for Chagas disease transmission to humans (4,36,37). In Guadalupe, the number of persons who slept in a room was the principal predictor of infestation and a determinant of vector population density. Simple interventions to decrease domestic triatomine populations in rural areas, such as keeping animals outside at night (38) and improving roofing materials (39), will likely have limited effect in Guadalupe. Although the presence of companion animals in rooms at night was associated with a 5-fold increase in vector density, animals were allowed to sleep inside in only 5% of rooms. Nearly all roofs in the community were of corrugated metal or other materials that do not provide refuge for triatomines. The only housing intervention that is likely to have a substantial effect against domiciliary triatomines in Guadalupe is completely stuccoing rooms. Schofield and Marsden showed that completely stuccoing a house could eliminate T. infestans from the human dwelling within 3 years (40). In Guadalupe, stuccoed rooms were only a fourth as likely to be infested and harbored a tenth the population of vectors compared with rooms of other materials. However, stuccoing must be complete to be effective; rooms in which mortar was used to fill the gaps between brick, sillar, and basalt were significantly more likely to be infested with the vector than rooms of other types.
Our study had several limitations. Triatomine collection was dependent on the excito-repellant effect of deltamethrin spray. In some materials, especially the unmortared basalt of the hillside, insecticide did not reach all refuges of triatomines, and our vector collections may have been incomplete. T. infestans can survive for many months without feeding, and insect population density may be more influenced by past, rather than present, host populations (4). We did not have information to evaluate the effect of previous animal populations on size of T. infestans populations at the time of spraying. Identification of households with triatomines infected with T. cruzi was limited by the number of insects captured at each site of collection, and the number of insects examined varied between households. The power of the analyses of estimated vector densities was diminished because we considered only 1 enclosure and room per household to maintain independence of observations.
Spatial analysis suggests that while the vector is distributed across Guadalupe, Chagas disease transmission is likely to be clustered. Households with T. cruzi–infected triatomines showed significant clustering. Many aspects of the complicated periurban ecology of reservoir, vector, and parasite populations could lead to spatial clustering of T. cruzi without clustering of its vector, but the most parsimonious explanation is a basic difference in the speed of vector and parasite dispersion. Guadalupe is a young community; 81% of households were constructed in the past 20 years. While triatomines may have had sufficient time to infest and colonize most suitable habitats in Guadalupe and may be considered endemic, T. cruzi may still be spreading from 1 or multiple points of introduction in a more epidemic fashion.
Many communities similar to Guadalupe are awaiting insecticide application. Acute cases of Chagas disease have been reported from communities in different parts of the city (Arequipa Regional Ministry of Health, unpub. data), though transmission of T. cruzi in these areas is likely focal. Timely, coordinated insecticide application is imperative to control Chagas disease in southern Peru and must be accompanied by effective surveillance for vector reinfestation. Improvement of peridomestic small animal enclosures with materials refractory to triatomine infestation could greatly increase the likelihood of eliminating the vector from the city.
Mr Levy is a graduate student in population biology, ecology, and evolution at Emory University and a visiting researcher at the Entomology Branch of the Centers for Disease Control and Prevention. His research interests include the epidemiology and ecology of parasitic diseases.
Acknowledgments
We are grateful to the community of Guadalupe for their participation and hospitality during this study. We especially thank the spray brigade and field collectors for their help; Jamie Maguire for his comments on the manuscript; Bob Wirtz, Ellen Dotson, and Ricardo Gürtler for their valuable input on study design; and Jenica Pastor and Fernando Malaga for their technical assistance.
M.Z.L. is supported by a Howard Hughes predoctoral fellowship. N.M.B. was supported by a Fogarty/Ellison fellowship and National Institutes of Health training grant 5T35AI007646-03. Additional support came from National Institutes of Health opportunity grant U19-AI-33061.
References
- World Health Organization. The World Health Report 2003, annex 2. Deaths by cause, sex and mortality stratum in WHO regions, estimates for 2002. 2003 [cited 2005 Nov 21]. Available from http://www.who.int/whr/2003/en/Annex2-en.pdf
- Kirchhoff LV, Weiss LM, Wittner M, Tanowitz HB. Parasitic diseases of the heart. Front Biosci. 2004;9:706–23. DOIPubMedGoogle Scholar
- Zeledon R, Rabinovich JE. Chagas' disease: an ecological appraisal with special emphasis on its insect vectors. Annu Rev Entomol. 1981;26:101–33. DOIPubMedGoogle Scholar
- Cohen JE, Gürtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–8. DOIPubMedGoogle Scholar
- Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–12. DOIPubMedGoogle Scholar
- Schofield CJ, Dias JC. The Southern Cone Initiative against Chagas disease. Adv Parasitol. 1999;42:1–27. DOIPubMedGoogle Scholar
- Lorca M, Garcia A, Contreras MC, Schenone H, Rojas A. Evaluation of a Triatoma infestans elimination program by the decrease of Trypanosoma cruzi infection frequency in children younger than 10 years, Chile, 1991–1998. Am J Trop Med Hyg. 2001;65:861–4.PubMedGoogle Scholar
- Silveira A, Vinhaes M. Elimination of vector-borne transmission of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94(Suppl 1):405–11. DOIPubMedGoogle Scholar
- Gajate P, Pietrokovsky S, Abramo Orrego L, Perez O, Monte A, Belmonte J, Triatoma infestans in greater Buenos Aires, Argentina. Mem Inst Oswaldo Cruz. 2001;96:473–7. DOIPubMedGoogle Scholar
- Albarracin-Veizaga H, de Carvalho ME, Nascimento EM, Rodrigues VL, Casanova C, Barata JM. Chagas disease in an area of recent occupation in Cochabamba, Bolivia. Rev Saude Publica. 1999;33:230–6. DOIPubMedGoogle Scholar
- Vallve SL, Rojo H, Wisnivesky-Colli C. Urban ecology of Triatoma infestans in San Juan, Argentina. Mem Inst Oswaldo Cruz. 1996;91:405–8. DOIPubMedGoogle Scholar
- Zeledon R, Calvo N, Montenegro VM, Lorosa ES, Arevalo C. A survey on Triatoma dimidiata in an urban area of the province of Heredia, Costa Rica. Mem Inst Oswaldo Cruz. 2005;100:507–12. DOIPubMedGoogle Scholar
- Ramsey JM, Alvear AL, Ordonez R, Munoz G, Garcia A, Lopez R, Risk factors associated with house infestation by the Chagas disease vector Triatoma pallidipennis in Cuernavaca metropolitan area, Mexico. Med Vet Entomol. 2005;19:219–28. DOIPubMedGoogle Scholar
- Pease GYF. Breve historia contemporánea del Perú. 2nd ed. México: Fondo de Cultura Económica; 1999.
- Gürtler RE, Cohen JE, Cecere MC, Lauricella MA, Chuit R, Segura EL. Influence of humans and domestic animals on the household prevalence of Trypanosoma cruzi in Triatoma infestans populations in northwest Argentina. Am J Trop Med Hyg. 1998;58:748–58.PubMedGoogle Scholar
- Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: Wiley; 2000.
- Thomson MC, Connor SJ, D'Alessandro U, Rowlingson B, Diggle P, Cresswell M, Predicting malaria infection in Gambian children from satellite data and bed net use surveys: the importance of spatial correlation in the interpretation of results. Am J Trop Med Hyg. 1999;61:2–8.PubMedGoogle Scholar
- Lee AH, Wang K, Yau KK, Somerford PJ. Truncated negative binomial mixed regression modelling of ischaemic stroke hospitalizations. Stat Med. 2003;22:1129–39. DOIPubMedGoogle Scholar
- Ripley B. The second-order analysis of stationary point processes. J Appl Probab. 1976;13:255–66. DOIGoogle Scholar
- Diggle PJ, Chetwynd AG. Second-order analysis of spatial clustering for inhomogeneous populations. Biometrics. 1991;47:1155–63. DOIPubMedGoogle Scholar
- Waller LA, Gotway CA. Applied spatial statistics for public health data. Hoboken (NJ): John Wiley & Sons; 2004.
- Getis A. Interaction modeling using second-order analysis. Environment and Planning. 1984;A16:173–83. DOIGoogle Scholar
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am J Trop Med Hyg. 2004;71:803–10.PubMedGoogle Scholar
- Vazquez-Prokopec GM, Ceballos LA, Kitron U, Gürtler RE. Active dispersal of natural populations of Triatoma infestans (Hemiptera: Reduviidae) in rural northwestern Argentina. J Med Entomol. 2004;41:614–21. DOIPubMedGoogle Scholar
- Vazquez-Prokopec GM, Cecere MC, Canale DM, Gürtler RE, Kitron U. Spatiotemporal patterns of reinfestation by Triatoma guasayana (Hemiptera: Reduviidae) in a rural community of northwestern Argentina. J Med Entomol. 2005;42:571–81. DOIPubMedGoogle Scholar
- Cecere MC, Gürtler RE, Canale D, Chuit R, Cohen JE. The role of the peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Rev Panam Salud Publica. 1997;1:273–9. DOIPubMedGoogle Scholar
- Salinas M. Crianza y comercialización de cuyes. Lima (Peru): Ediciones Ripalme; 2002.
- Herrer A. Importancia del cobayo como reservoriode la enfermedad de Chagas en la región sudoccidental. Rev Med Exp. 1955;9:45–55.
- Acosta HM, Ferreira CS, de Carvalho ME. Human infection with Trypanosoma cruzi in Nasca, Peru: a seroepidemiological survey. Rev Inst Med Trop Sao Paulo. 1997;39:107–12. DOIPubMedGoogle Scholar
- Cordova E. Investigation of Chagas disease in Peru. Epidemiological study in the Tambo. valley (Matalaque district. Department of Moquegua). I. Preliminary observations. 1958–1959. Bol Chil Parasitol. 1961;16:54–9.PubMedGoogle Scholar
- Schofield CJ. Density regulation of domestic populations of Triatoma infestans in Brazil. Trans R Soc Trop Med Hyg. 1980;74:761–9. DOIPubMedGoogle Scholar
- Schofield CJ. The role of blood intake in density regulation of populations of Triatoma infestans (Klug) (Hemiptera: Reduviidae). Bull Entomol Res. 1982;72:617–29. DOIGoogle Scholar
- Schofield CJ. Nutritional status of domestic populations of Triatoma infestans. Trans R Soc Trop Med Hyg. 1980;74:770–8. DOIPubMedGoogle Scholar
- Lehane MJ, Schofield CJ. Field experiments of dispersive flight by Triatoma infestans. Trans R Soc Trop Med Hyg. 1981;75:399–400. DOIPubMedGoogle Scholar
- Cecere MC, Gürtler RE, Chuit R, Cohen JE. Effects of chickens on the prevalence of infestation and population density of Triatoma infestans in rural houses of north-west Argentina. Med Vet Entomol. 1997;11:383–8. DOIPubMedGoogle Scholar
- Rabinovich JE, Wisnivesky-Colli C, Solarz ND, Gürtler RE. Probability of transmission of Chagas disease by Triatoma infestans (Hemiptera: Reduviidae) in an endemic area of Santiago del Estero, Argentina. Bull World Health Organ. 1990;68:737–46.PubMedGoogle Scholar
- Gürtler RE, Chuit R, Cecere MC, Castanera MB, Cohen JE, Segura EL. Household prevalence of seropositivity for Trypanosoma cruzi in three rural villages in northwest Argentina: environmental, demographic, and entomologic associations. Am J Trop Med Hyg. 1998;59:741–9.PubMedGoogle Scholar
- Cecere MC, Gürtler RE, Chuit R, Cohen JE. Factors limiting the domestic density of Triatoma infestans in north-west Argentina: a longitudinal study. Bull World Health Organ. 1998;76:373–84.PubMedGoogle Scholar
- Schofield CJ, Marsden PD. The effect of wall plaster on a domestic population of Triatoma infestans. Bull Pan Am Health Organ. 1982;16:356–60.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 12, Number 9—September 2006
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Michael Z. Levy, Centers for Disease Control and Prevention, 4770 Buford Hwy, Mailstop F42, Atlanta, GA 30341-3724, USA
Top