Volume 12, Number 2—February 2006
Perspective
Antimicrobial Drug Resistance, Regulation, and Research1
Figure 3
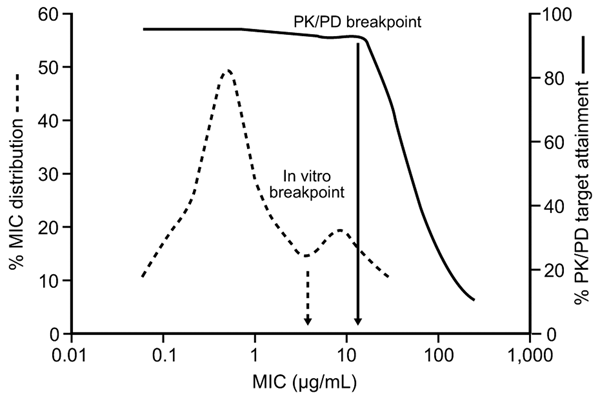
Figure 3. Relationship between MIC and attainment of the pharmacokinetic/pharmacodynamic (PK/PD) target for effect. Accumulating evidence supports the use of separate PK/PD breakpoints for clinical decision making, distinct from in vitro breakpoints used for epidemiologic surveillance. A breakpoint derived from PK/PD data represents the highest MIC for which the unbound plasma concentrations of the drug (after standard doses) are sufficient to achieve the target PK/PD exposure.
1This article is based on presentations and discussions held at the Second Colloquium of the International Forum on Antibiotic Resistance (IFAR), held on September 13, 2003, in Chicago, Illinois, USA. IFAR is a multidisciplinary, international group concerned with evaluating current knowledge regarding antimicrobial drug resistance and the means for its control. This article represents the opinions of the participants at the second IFAR colloquium and not necessarily those of the institutions for whom they work.