Volume 12, Number 2—February 2006
Perspective
Antimicrobial Drug Resistance, Regulation, and Research1
Figure 4
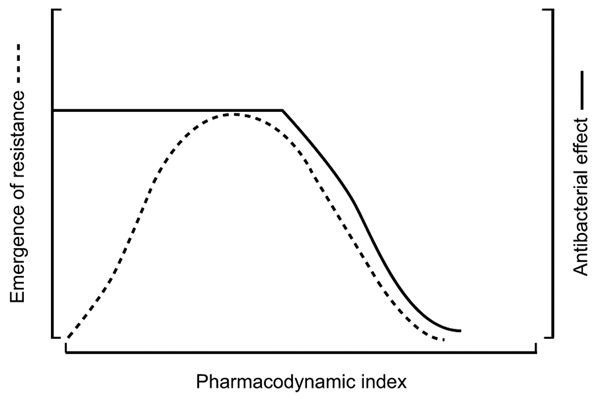
Figure 4. Relationship between the dominant pharmacokinetic/pharmacodynamic (PK/PD) index, efficacy, and resistance emergence in vitro (both quantified by the number of bacterial colony-forming units). The PK/PD index is related to efficacy in a sigmoid curve and the resistance emergence by an inverted U-shaped curve (21).
References
- Commission of the European Communities. Communication from the commission on a community strategy against antimicrobial resistance. Brussels: The Commission; 2001.
- Interagency Task Force on Antimicrobial Resistance. Public health action plan to combat antimicrobial resistance. Atlanta: Centers for Disease Control and Prevention; 2001.
- World Health Organization. WHO global strategy for the containment of antimicrobial resistance. Geneva: The Organization; 2001.
- Powers JH. Antimicrobial drug development—the past, present and future. Clin Microbiol Infect. 2004;10(Suppl 4):23–31. DOIPubMedGoogle Scholar
- Infectious Diseases Society of America. Bad bugs, no drugs. Alexandria (VA): The Society; 2004.
- Nordberg P, Monnet DL, Cars O. Antibacterial resistance. Background document for the WHO project: priority medicines for Europe and the World—a public health approach to innovation. 2005 Aug 9 [cited 2005 Nov 22]. Available from http://mednet3.who.int/prioritymeds/report/index.htm
- Norrby SR, Nord CE, Finch R. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005;5:115–9.PubMedGoogle Scholar
- Metlay JM, Singer DE. Outcomes in lower respiratory tract infections and the impact of antimicrobial drug resistance. Clin Microbiol Infect. 2002;8(Suppl 2):1–11. DOIPubMedGoogle Scholar
- Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry. E9 statistical principles for clinical trials. Rockville (MD): The Administration; 1998.
- Dudley MN, Ambrose PG. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr Opin Microbiol. 2000;3:515–21. DOIPubMedGoogle Scholar
- Andes D, Craig WA. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents. 2002;19:261–8. DOIPubMedGoogle Scholar
- MacGowan A, Bowker K. Developments in PK/PD: optimising efficacy and prevention of resistance. A critical review of PK/PD in in vitro models. Int J Antimicrob Agents. 2002;19:291–8. DOIPubMedGoogle Scholar
- MacGowan AP. Elements of design: the knowledge on which we build. Clin Microbiol Infect. 2004;10(Suppl 2):6–11. DOIPubMedGoogle Scholar
- MacGowan A, Rogers C, Holt A, Wootton M, Bowker K. Assessment of different antibacterial effect measures used in in vitro models of infection and subsequent use in pharmacodynamic correlations for moxifloxacin. J Antimicrob Chemother. 2000;46:73–8. DOIPubMedGoogle Scholar
- Craig WA, Kiem S, Andes D, Ambrose P, Jones R. Impact of ESBLs on in vivo activity of four cephalosporins in the neutropenic mouse-thigh infection model [abstract A-1318]. In: Abstracts of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago; 2003 Sep 14–17. Washington; American Society for Microbiology; 2003.
- Jumbe N, Louie A, Leary R, Liu W, Deziel MR, Tam VH, Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J Clin Invest. 2003;112:275–85.PubMedGoogle Scholar
- Bradley JS, Dudley MN, Drusano GL. Predicting efficacy of antiinfectives with pharmacodynamics and Monte Carlo simulation. Pediatr Infect Dis J. 2003;22:982–92. DOIPubMedGoogle Scholar
- Dudley MN. Commentary on dual individualization with antibiotics. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied pharmacokinetics-principles of therapeutic drug monitoring. 3rd ed. Vancouver (WA): Applied Therapeutics; 1992. p. 18-1–18-13.
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Eleventh informational supplement. NCCLS Document M100-S11. Wayne (PA): The Committee; 2001.
- Ambrose PG, Bhavnani SM, Jones RN, Jones RN, Craig WA, Dudley MN. Use of pharmacokinetics-pharmacodynamics and Monte Carlo simulation as decision support for the re-evaluation of NCCLS cephem susceptibility breakpoints for Enterobacteriaceae [abstract A-138]. In: Abstracts of the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington; 2004 Oct 30-Nov 2. Washington; American Society for Microbiology; 2004.
- Craig WA, Kiem S. Pharmacodynamic requirements to prevent the emergence of quinolone-resistant Streptococcus pneumoniae in an animal model [abstract 81]. In: Abstracts of the 40th Infectious Diseases Society of America; Chicago; 2002 Oct 24–27; Alexandria (VA): Infectious Disease Society of America.
- Knudsen JD, Odenholt I, Erlendsdottir H, Gottfredsson M, Cars O, Frimodt-Moller N, Selection of resistant Streptococcus pneumoniae during penicillin treatment in vitro and in three animal models. Antimicrob Agents Chemother. 2003;47:2499–506. DOIPubMedGoogle Scholar
- Odenholt I, Gustafsson I, Lowdin E, Cars O. Suboptimal antibiotic dosage as a risk factor for selection of penicillin-resistant Streptococcus pneumoniae: in vitro kinetic model. Antimicrob Agents Chemother. 2003;47:518–23. DOIPubMedGoogle Scholar
- Zinner S, Gilbert DS, Simmons K, Lubenko I, Zhao X, Drlica K, Emergence of resistant Streptococcus pneumoniae in an in vitro dynamic model that simulates moxifloxacin concentrations in and out of the mutant selection window: related changes in susceptibility and resistance frequency [abstract A-1149]. In: Abstracts of the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago; 2003 Sep 14–17. Washington: American Society for Microbiology; 2003.
- Blaser J, Stone BB, Groner MC, Zinner S. Comparative study with enoxacin and netilmicin in pharmacodynamic model to determine importance of the ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1054–60.PubMedGoogle Scholar
- MacGowan AP, Rogers CA, Holt HA, Bowker KE. Activities of moxifloxacin against, and emergence of resistance in, Streptococcus pneumoniae and Pseudomonas aeruginosa in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 2003;47:1088–95. DOIPubMedGoogle Scholar
- Thomas JK, Forrest A, Bhavnani SM, Hyatt JM, Cheng A, Ballow CH, Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother. 1998;42:521–7.PubMedGoogle Scholar
- Pallares R, Linares J, Vadillo M, Cabellos C, Manresa F, Viladrich PF, Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–80. DOIPubMedGoogle Scholar
- Yu VL, Chiou CC, Feldman C, Ortqvist A, Rello J, Morris AJ, An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37:230–7. DOIPubMedGoogle Scholar
- Powers JH, Moncada V, Johann-Liang R. Disease severity (DS) assessment in community-acquired pneumonia (CAP) antimicrobial clinical trials: a comparison of the PORT criteria with the original and revised ATS criteria [abstract L-655]. In: Abstracts of the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington; 2004 Oct 30–Nov 2. Washington: American Society for Microbiology; 2004.
- Ambrose PG, Anon JB, Owen JS, Wan Wart S, McPhee ME, Bhavnani SM, Use of pharmacokinetic endpoints in the evaluation of gatifloxacin for the treatment of acute maxillary sinusitis. Clin Infect Dis. 2004;38:1513–20. DOIPubMedGoogle Scholar
- Committee for Proprietary Medicinal Products. Note for guidance on evaluation of medicinal products for the treatment of bacterial infection. Document CPMP/EWP/558/95. London: European Agency for the Evaluation of Medicinal Products; 2004.
- Committee for Proprietary Medicinal Products. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. Document CPMP/EWP/2655/99. London: European Agency for the Evaluation of Medicinal Products; 2000.
- Committee for Proprietary Medicinal Products. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. Document CPMP/EWP/2655/99. London: European Agency for the Evaluation of Medicinal Products; 2000.
- Turnidge JD, Bell JM; Sentry Asia-Pacific Participants. Reduced quinolone susceptibility is common in Salmonella species from the Asia-Pacific region: results from the Sentry Asia-Pacific Surveillance program 2001 [abstract C2-1284]. In: Abstracts of the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Diego; 2002 Sep 27–30. Washington: American Society for Microbiology; 2002.
- McCaig LF, Besser RE, Hughes JM. Antimicrobial drug prescription in ambulatory care settings, United States, 1992–2000. Emerg Infect Dis. 2003;9:432–7.PubMedGoogle Scholar
- Beilby J, Marley J, Walker D, Chamberlain N, Burke M; FIESTA Study Group. Effect of changes in antibiotic prescribing on patient outcomes in a community setting: a natural experiment in Australia. Clin Infect Dis. 2002;34:55–64. DOIPubMedGoogle Scholar
- Food and Drug Administration. Labeling requirements for systemic antibacterial drug products intended for human use. Document 21CFR, part 201. Rockville (MD): The Administration; 2003.
1This article is based on presentations and discussions held at the Second Colloquium of the International Forum on Antibiotic Resistance (IFAR), held on September 13, 2003, in Chicago, Illinois, USA. IFAR is a multidisciplinary, international group concerned with evaluating current knowledge regarding antimicrobial drug resistance and the means for its control. This article represents the opinions of the participants at the second IFAR colloquium and not necessarily those of the institutions for whom they work.