Volume 12, Number 6—June 2006
Dispatch
Hantaviruses in Serbia and Montenegro
Abstract
Hantaviruses are endemic in the Balkan Peninsula. An outbreak of hemorrhagic fever with renal syndrome occurred in 2002 in Serbia and Montenegro. The epidemiologic characteristics and genetic relatedness of Dobrava/Belgrade virus strains responsible for most cases are described.
Hantaviruses (Bunyaviridae) are enveloped, single-stranded, negative-sense RNA viruses with a tripartite genome consisting of a small (S), a medium (M), and a large (L) segment, which encode the nucleocapsid protein, the glycoprotein precursor and the putative RNA polymerase, respectively (1). Hantaviruses are transmitted to humans through aerosols of excreta from small mammals, mainly rodents, that have had silent lifelong-infections. More than 30 different hantaviruses have been distinguished so far, at least half are related to disease in humans. These viruses cause hemorrhagic fever with renal syndrome (HFRS) in Asia and Europe and hantavirus pulmonary syndrome (HPS) in America. HFRS is caused by Hantaan (HTNV), Dobrava/Belgrade (DOBV), Seoul (SEOV), and Puumala (PUUV) hantaviruses, while HPS is caused by Sin Nombre (SNV) and related viruses. Each hantavirus is associated with a specific primary rodent reservoir of the Muridae family; these relationships have coevolved over a long period, probably >50 million years (1).
HFRS is endemic in the Balkan Peninsula, where sporadic cases or outbreaks have been reported. The disease is seen during the summer and affects mainly adults (2,3), although infections in children, some fatal (4), have been reported. Hantaviruses associated with disease in humans in Balkans are DOBV, carried by the yellow-necked mouse (Apodemus flavicollis), which causes severe HFRS with a fatality rate up to 10%, and PUUV, carried by the red bank vole (Clethrionomys glareolus). PUUV causes nephropathia epidemica, a milder form of HFRS, with a fatality rate <1% (3,5–8). Recently, A. agrarius was found to be an additional host of DOBV, causing a milder disease than that associated with A. flavicollis (9). Additionally, Tula virus RNA was amplified from lung tissues of a European pine vole (Pitymys subterraneus) in Serbia (10).
The first probable HFRS case was reported in former Yugoslavia in 1952 (11,12); the first identified epidemic of HFRS occurred in 1961 (13). Some years (namely, 1961, 1967, 1979, 1986, 1989, and 1995 [2]) are characterized by increased HFRS cases. Different factors, such as weather and food abundance, could influence the dynamics of rodent populations.
The more recent large epidemic in Serbia and Montenegro occurred in 2002 with 128 laboratory-confirmed cases. The number of confirmed cases was lower in the following years. In 2003, 16 cases occurred in Serbia and 18 in Montenegro (1 fatal). In 2004, 20 cases (1 fatal) occurred in Serbia and 11 in Montenegro.
During 2002, a total of 376 serum samples from patients with suspected HFRS cases were tested in Torlak Institute, Belgrade, by indirect immunofluorescent assay (IFA) for the presence of hantavirus antibodies. IFA was performed on spot slides containing Vero E6 cells infected with HTNV, SEOV, PUUV, and DOBV. For 128 cases (77 from Serbia, 51 from Montenegro), a laboratory diagnosis of HFRS was made. Most patients (77.3%) were infected with DOBV-like viruses; the rest were infected with PUUV-like viruses. Briefly, 53 (69%) of 77 samples from Serbia and 46 (90%) of 51 from Montenegro had higher antibody titers to HTNV and DOBV than to PUUV; the other samples had higher titers to PUUV. Two Serbian patients who lived in Leskovac died. Most DOBV-like infections from Serbia occurred in the south (Leskovac, Vranje, Nis, Surdulica, Vlasina), while the PUUV-like infections occurred in the north (Vojvodina and area near the River Drina) (map of Serbia and Montenegro available from http://www.un.org/Depts/Cartographic/map/profile/yugoslav.pdf).
Thirty-one serum samples from the IFA-positive patients were sent to Aristotle University for additional testing. Samples were taken from 21 HFRS patients with a mean age of 40.3 years (21–68 years); 1 sample was obtained from a 5-month-old male infant, whose mother had HFRS at the time of delivery. Two of 21 patients died. Enzyme-linked immunosorbent assay (ELISA) to detect immunoglobulin G (IgG) and IgM antibodies to HTNV and PUUV was performed with kits by Progen (Biotechnik GmbH, Heidelberg, Germany). IgM antibodies to HTNV were detected in 18 of 21 patients; 9 also carried IgM antibodies to PUUV, although in lower titers than to HTNV (Table 1). IgG antibodies to HTNV were present in 17 of 21 patients; in 3 patients low titers of IgM antibodies to PUUV were also detected. The infant had IgG antibodies to HTNV. In 1 sample (DR) no antibodies to HTNV or PUUV were detected, although it was positive by IFA. ELISA results suggested that all 21 patients had an HTNV-like infection.
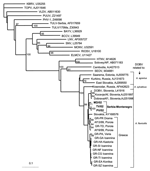
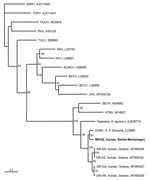
Viral RNA was extracted from IgM-positive samples (a sample from the neonate was also included) by using the viral RNA extraction kit (Qiagen GmbH, Hilden, Germany). Reverse transcription and nested amplification were performed with primers previously designed to detect partial S and M segment sequences from hantaviruses associated with rodents of the Murinae and Arvicolinae subfamilies (14,15). Three samples (M.D., T.V., P.V.) gave a PCR product of the expected size of 599 bp, when primers specific for the S segment of hantaviruses associated with Murinae rodents were used; 1 sample (MD) gave a product of 317 bp with the primers for the M segment of the same hantaviruses. No product was obtained when primers specific for the S segment of hantaviruses associated with Arvicolinae rodents were used. Nucleotide sequences were aligned with respective hantavirus sequences retrieved from GenBank; genetic distances were measured by the neighbor-joining method, and phylogenetic trees were constructed by using PHYLIP (Phylogeny Inference Package by J. Felsenstein [http://evolution.genetics.washington.edu/phylip.html]). The nucleotide sequences were assigned the accession numbers DQ305279-DQ305282.
Two phylogenetic trees were constructed, one for the S segment (Figure 1) and another for the M segment (Figure 2). In both trees, hantavirus strains from Serbia and Montenegro cluster with other DOBV sequences and were associated with the rodent A. flavicollis. In the S segment tree, sequences of this study comprise the Serbian clade in the DOBV-A. flavicollis cluster. In the same cluster are the Slovenian, Slovakian, and Greek clades. Sequences of this study differ by 0.3%–2.6% at the nucleotide level, with identical deduced aminoacid sequences. Genetic distances with other DOBV sequences are seen in Table 2. Concerning the M segment, a fragment of the G1-coding region of patient MD differed by 5.7% at the nucleotide level from the Slovenian DOBV strain isolated from A. flavicollis, with identical deduced amino acid sequences. The differences from DOBV strains from northwestern Greece were 8.5%–9.4% and 1% at nucleotide and amino acid levels, respectively.
Patient TV was a 38-year-old woman who lived in Vranje. Patient PV was a 29-year-old woman who lived in Leskovac. Both of these locations are in southeastern Serbia. PV died on day 6 of illness. Patient MD was living in Beograd. However, his sequences were similar to those of patients TV and PV. His travel history showed that 18 days before the onset of illness, he was on vacation in Kolasin Mountain in Montenegro, where he was probably infected. Thus, all sequences of this study were from the southern region of the country and clustered with other DOBV strains associated with A. flavicollis rodents. However, the involvement of other hantaviruses in the outbreak cannot be excluded.
Although the number of samples tested was limited, this study gives the first genetic information on DOBV strains circulating in Serbia and Montenegro. Further studies of both patients and small mammals in the region are needed to find out the exact epidemiology of HFRS in the country.
Dr Papa is assistant professor of medicine and head of the molecular diagnostics and special pathogens laboratory at the Department of Microbiology, School of Medicine, Aristotle University of Thessaloniki, Greece. Her major interest is the molecular epidemiology of hantaviruses and nairoviruses.
Acknowledgment
We thank Vassiliki Pavlidou and Panagiota Papadopoulou for excellent technical assistance.
References
- Nichol ST, Ksiazek TG, Rollin PE, Peters CJ. Hantavirus pulmonary syndrome and newly described hantaviruses in the United States. In: Elliott RM, editor. The Bunyaviridae. New York: Plenum Press; 1996. p. 269–80.
- Avšič-Županc T. Hantaviruses and hemorrhagic fever with renal syndrome in the Balkans. In: Saluzzo JF, Dodet B, editors. Factors in the emergence and control of rodent-borne viral diseases. Paris: Elsevier SAS; 1999. p. 93–8.
- Antoniadis A, Stylianakis A, Papa A, Alexiou-Daniel S, Lampropoulos A, Nichol ST, Direct genetic detection of Dobrava virus in Greek and Albanian patients with hemorrhagic fever with renal syndrome. J Infect Dis. 1996;174:407–10. DOIPubMedGoogle Scholar
- Peco-Antic A, Popovic-Rolovic M, Gligic A, Popovic D, Jovanovic O, Kostic M. Clinical characteristics of haemorrhagic fever with renal syndrome in children. Pediatr Nephrol. 1992;6:335–8. DOIPubMedGoogle Scholar
- Glicic A, Dimkovic N, Xiao SY, Buckle GJ, Jovanovic D, Velimirovic D, Belgrade virus: a new hantavirus causing severe hemorrhagic fever with renal syndrome in Yugoslavia. J Infect Dis. 1992;166:113–20. DOIPubMedGoogle Scholar
- Avšič-Županc T, Xiao SY, Stojanovic R, Gligic A, van der Groen G, LeDuc JW. Characterization of Dobrava virus: a Hantavirus from Slovenia, Yugoslavia. J Med Virol. 1992;38:132–7. DOIPubMedGoogle Scholar
- Lundkvist A, Hukic M, Hörling J, Gilljam M, Nichol S, Niklasson B. Puumala and Dobrava viruses cause haemorrhagic fever with renal syndrome (HFRS) in Bosnia-Herzegovina: evidence of highly cross-neutralizing antibody responses in early patient sera. J Med Virol. 1997;53:51–9. DOIPubMedGoogle Scholar
- Markotic A, Nichol ST, Kuzman I, Sanchez AJ, Ksiazek TG, Gagro A, Characteristics of Puumala and Dobrava infections in Croatia. J Med Virol. 2002;66:542–51. DOIPubMedGoogle Scholar
- Avšič-Županc T, Nemirov K, Petrovec M, Trilar T, Poljak M, Vaheri A, Genetic analysis of wild-type Dobrava hantavirus in Slovenia: co-existence of two distinct genetic lineages within the same natural focus. J Gen Virol. 2000;81:1747–55.PubMedGoogle Scholar
- Song JW, Gligic A, Yanagihara R. Identification of Tula hantavirus in Pitymys subterraneus captured in the Cacak region of Serbia-Yugoslavia. Int J Infect Dis. 2002;6:31–6. DOIPubMedGoogle Scholar
- Simič M, Mirič V. Successful application of peritoneal dialysis in a case of renal insufficiency [in Serbian]. Vojnosanit Pregl. 1952;9:285–90.PubMedGoogle Scholar
- Radosevic Z, Mohacek I. The problem of nephropathia epidemica Myhrman-Zetterholm in relation to acute interstitial nephritis. Acta Med Scand. 1954;149:221–8. DOIPubMedGoogle Scholar
- Heneberg D, Vuksic L. Epidemic of hemorrhagic fever in certain workplaces in Fruska Gora [in Serbian]. Zbornik Vojnomedicinske Akademije. 1962;4:263–71.
- Bowen MD, Gelbmann W, Ksiasek TG, Nichol ST, Nowotny N. Puumala virus and two genetic variants of Tula virus are present in Austrian rodents. J Med Virol. 1997;53:174–81. DOIPubMedGoogle Scholar
- Papa A, Johnson AM, Stockton PC, Bowen MD, Spiropoulou CF, Alexiou-Daniel S, Retrospective serological and genetic study of the distribution of hantaviruses in Greece. J Med Virol. 1998;55:321–7. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 12, Number 6—June 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Anna Papa, A’ Dept. of Microbiology, School of Medicine, Aristotle University of Thessaloniki, 54006, Thessaloniki, Greece; email address:
Top